
Revisiting the impact of clinicopathologic characteristics in PD-L1 profile in a large cohort of non-small cell lung cancer
Highlight box
Key findings
• Programmed cell death ligand-1 (PD-L1) expression is associated with adverse clinicopathological factors.
• Lower metastasis is associated with high PD-L1 expression of SP142 in tumor-infiltrating immune cells (ICs).
What is known and what is new?
• Previous studies on the clinicopathological significance of PD-L1 expression have shown conflicting results, and association with metastasis remains unclear in non-small cell carcinoma.
• We revealed that PD-L1 expression is associated with several clinicopathological factors, and also revealed an inverse relationship between PD-L1 expression of SP142 in tumor-infiltrating ICs and metastasis.
What is the implication, and what should change now?
• As PD-L1 expression in non-small cell lung cancer (NSCLC) is associated with adverse clinicopathological features, it could be utilized to predict poor prognosis.
• PD-L1 expression of SP142 in tumor-infiltrating ICs could be a potential marker for low metastasis.
• These findings could help in further establishing criteria for identifying responders and non-responders to anti-PD-L1 therapy and guide treatment approaches.
Introduction
Lung cancer is the most common cause of deaths from cancer and the second most commonly diagnosed cancer worldwide (1). Surgical resection is the standard modality for treatment; however, many cases, especially those in the advanced stage or with metastasis and recurrence, require additional treatment. The next choice is chemotherapy, which has some limitations. Therefore, many studies have focused on developing alternative treatments, including targeted therapy. Despite these efforts, lung cancer treatment remains challenging.
Cancer immunotherapy has emerged as a powerful new therapy after traditional systemic cytotoxic chemotherapy, which has reached its limit of benefit. In particular, T cell checkpoint blockade agents have revealed remarkable treatment responses in some solid cancers, including melanoma and non-small cell lung cancer (NSCLC) (2,3). Several immune-checkpoint inhibitor drugs are continually undergoing development for the treatment of NSCLC. Recently, immunotherapies targeting the programmed cell death ligand-1 (PD-L1) and programmed cell death-1 (PD-1) pathways have shown remarkable outcomes, increasing progression-free and overall survival rates (4-7). The Food and Drug Administration (FDA) has approved various PD-L1/PD-1 inhibitors, T-cell checkpoint blockade drugs, and representative drugs such as nivolumab, pembrolizumab, durvalumab, atezolizumab, and avelumab (2). According to the National Comprehensive Cancer Network (NCCN) guideline revised in 2017, pembrolizumab (PD-1 inhibitor) is recommended as the first-line treatment when PD-L1 expression is positive (more than 50%) and EGFR, ALK, and ROS1 are negative in the histological classification of lung cancer (8,9).
Although PD-L1/PD-1 inhibitors are commonly considered for clinical use, they are limited in terms of cost and stability. Therefore, immunotherapy cannot be used in all cancer cases, and the decision on whether patients comply with the indications should be made through a test to ensure stability and treatment response before initiating therapy. Immunohistochemical staining of samples obtained from patients is a cost-effective technique and is widely used in the daily practice of pathology; therefore, it is usually used as a reference point for detecting PD-L1 positivity. Each immunotherapeutic drug has a paired companion diagnostic, requiring different cutoff values among diverse immunohistochemical assays against tumor cells (TCs) and tumor-infiltrating immune cells (ICs), including Dako 28-8 pharmDx, Dako 22C3 pharmDx, Ventana SP142, and Ventana SP263 (10,11).
The cutoff value of each assay is determined based on numerous studies that investigated the correlation between PD-L1 expression and response to immunotherapy. Taube et al. revealed that PD-L1 positivity, defined as ≥5% positive TC, in 41 cases, including 16 melanoma, 12 NSCLC, 6 colorectal cancer, 5 renal cell cancer, and 2 prostate cancer, correlated well with the clinical response (12). Carbognin et al. analyzed 20 clinical trials of approximately 1,500 melanoma and non-small cell carcinoma cases, and revealed that a cutoff value of 5% of TC showed better prediction than a cutoff value of 1% (13). However, only a subset of cases with PD-L1 expression show an actual treatment response to immunotherapy, while some cases with negative PD-L1 expression also demonstrate a treatment response to the drugs, revealing an uncertain correlation between the immunohistochemical assay and the actual response (4,13-17). In addition, the actual treatment response to anti-PD-L1 agents is now assumed to be more complicated than previously thought owing to the tumor microenvironment and tumor heterogeneity (16,18). Furthermore, each cutoff value has been utilized for various PD-L1 assays as a criterion for the use of anti-PD-L1 agents; however, these cutoff values have been evaluated to disregard the immunological properties of patients, thus failing to separate responders from non-responders (16,19).
As cutoff values for PD-L1 expression have revealed poor prediction for responsiveness to anti-PD-L1 agents, we assumed that immunohistochemical expression of PD-L1 might not be a single independence factor, but confounded by certain conventional clinicopathologic factors. If so, overall prognosis would better be predicted by investigating associated clinicopathologic factors which are already known for its significance on prognosis, rather than investigating PD-L1 expression alone, regardless of responsiveness to anti-PD-L1 agents. Similarly, many researchers have focused on clinicopathological factors associated with prognosis in NSCLC (20). However, studies on the clinicopathological significance of PD-L1 expression have shown various discrepancies. There have been conflicting reports of PD-L1 expression associated with unfavorable prognostic factors (16,18,21-27), favorable prognostic factors (28-31), and even mixed favorable and unfavorable prognostic factors (28,31). Due to these variable results, prognostic effect of PD-L1 expression has been considered to be unclear.
Therefore, we investigated the clinicopathological significance of PD-L1 overexpression in primary lung cancer using the most widely used clones, Ventana SP263 and SP142. We present this article in accordance with the REMARK reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-812/rc).
Methods
Sample collection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Korea University Anam Hospital (IRB No. 2022AN0558). The study was conducted in retrospective way, and informed consent was waived. Cases of primary lung cancer with PD-L1 assay, performed using needle biopsy specimens or surgical resection specimens at the Korea University Anam Hospital from 2017 to July 2021, were retrospectively collected. Pathological data were retrospectively reviewed from the hematoxylin and eosin (H&E)-stained slides, while clinical data were investigated through chart review. The follow-up period was from the initial diagnosis to death, follow-up loss, or end of follow up, September 2023.
The collected clinicopathological data included age, sex, tumor type, recurrence, metastasis, treatment method (operation, chemotherapy, radiation therapy, and observation), type of anticancer drug, use of PD-L1 inhibitor, survival, recurrence, metastasis, and last follow-up date. Prognosis was evaluated based on disease-free survival (DFS), set as the length of time in days from the date of treatment to the date of the last follow-up, or local or distant recurrence.
Additional pathological data collected from the resected cases included tumor size, adenocarcinoma subtype, pathological T stage, visceral pleural invasion, lymphatic invasion, venous invasion, and perineural invasion. Pathological T stage was assigned based on the 8th edition of American Joint Committee on Cancer (AJCC). Tumor size was defined as the largest tumor dimension (mm).
Immunochemistry and evaluation of PD-L1 expression
The formalin-fixed paraffin-embedded blocks were previously preserved at room temperature, in storage room of department of pathology, Korea University Anam Hospital. Tissue sections were cut in 4 µm of thickness. PD-L1 expression was assessed using SP142 (Ventana, USA) and SP263 (Ventana, USA) antibodies. PD-L1 immunohistochemistry was performed using the Ventana Benchmark Ultra automated staining system (Ventana Medical Systems, Tucson, AZ, USA) according to the standard manufacturing protocols. For evaluation of background staining, aa negative reagent control was also applied. Cell conditioning 1 at high pH (pH 8) was used for antigen retrieval, and the Ventana OptiView DAB IHC Detection Kit was used to detect the reaction product. For positive control, placental and tonsillar tissues were utilized for SP263 and SP142, respectively. The stained slides were evaluated via light microscopic examination, by the naked eyes of one expert pathologist.
Per Ventana’s interpretation guide for NSCLC (32,33), PD-L1 expression was evaluated. SP263 expression was measured as the percentage of TCs with membranous staining regardless of intensity. SP142 expression was measured in both the TC and IC areas. TC expression was scored as the percentage of TCs with membranous staining regardless of intensity. IC expression was scored as the proportion of tumor area that is occupied by PD-L1 staining ICs regardless of intensity. The IC areas were visually encircled as closely as possible, and combined to estimate the proportion of tumor area occupied by IC aggregates. The tumor area was defined as the area occupied by viable TCs, and their associated intra- and contiguous peritumoral stroma. The boundary of peritumor stroma was visually defined without specific distance criteria, since it was well distinguished from surrounding normal tissue. Patients with necrotic tumors were excluded from the study. The decision to include IC expression was previously determined by developers, on the basis of the clinical research (19). SP263 (Figure 1), SP 142 TC (Figure 2) and IC (Figure 3) expressions were categorized one of three categories (<1%, 1–49%, 50–100%). Each PD-L1 expression was classified as negative (expression <1%) or positive (expression ≥1%). Positive expression was further classified as low (1%≤ expression <50%) or high (expression ≥50%).
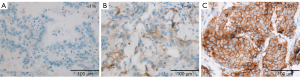
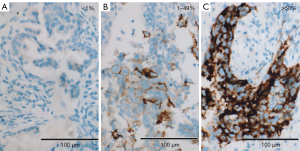
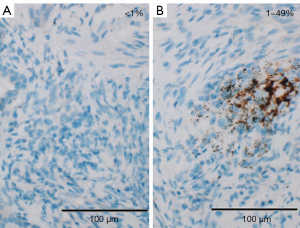
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics software (version 28.0; IBM, Boston, MA, USA). Correlations between PD-L1 expression and clinicopathological features of all cases, resection cases, and adenocarcinoma subtypes of resection cases were assessed using Fisher’s exact test. The odds ratios (ORs) between PD-L1 expression and clinicopathological features of all cases, resection cases, and the adenocarcinoma subtype of resection cases were assessed using logistic regression analysis. In logistic regression analysis, each parameter was verified by Wald test using backward elimination. The correlation between PD-L1 expression and DFS was evaluated using an independent sample t-test. P value under 0.05 was considered statistically significant. The concordance rate of PD-L1 expression between SP263 and SP142 antigens were evaluated using Cohen’s Kappa Coefficient.
Results
Clinicopathological characteristics of the cases
The clinicopathological data of all patients were collected retrospectively (Table 1). Of the 344 patients, 190 (55.2%) were biopsied, and 154 (44.8%) underwent resection. There were 140 cases (40.7%) who were younger than 70 years and 204 cases (59.3%) who were 70 years or older. The median age was 72 years for 223 male patients (range, 42–89 years), and 72 years for 121 female patients (range, 34–98 years). The tumor types were adenocarcinoma in 247 patients (71.8%), squamous cell carcinoma (SqCC) in 83 patients (24.1%), and NSCLC, not otherwise specified, in 14 patients (4.1%). PD-L1 inhibitor was used in 36 cases (10.5%). Recurrence was observed in 39 patients (11.3%). Metastasis was identified in 103 patients (29.9%). DFS averaged 1,399.1 days (46.0 months; range, 6–45,187 days). The median follow-up time was 719.5 days (23.6 months).
Table 1
| Clinicopathologic factors | Value, n (%) or mean (range) |
|---|---|
| Specimen type | |
| Biopsy | 190 (55.2) |
| Resection | 154 (44.8) |
| Age (years) | |
| <70 | 140 (40.7) |
| ≥70 | 204 (59.3) |
| Sex | |
| Male | 223 (64.8) |
| Female | 121 (35.2) |
| Tumor type | |
| Adenocarcinoma | 247 (71.8) |
| SqCC | 83 (24.1) |
| NOS | 14 (4.1) |
| Treatment | |
| Observation | 56 (16.3) |
| OP | 89 (25.9) |
| CTx | 40 (11.6) |
| RTx | 41 (11.9) |
| OP + CTx | 36 (10.5) |
| OP + RTx | 4 (1.2) |
| CTx + RTx | 54 (15.7) |
| OP + CTx + RTx | 24 (7.0) |
| PD-L1 inhibitor usage | |
| No | 311 (90.4) |
| Yes | 33 (9.6) |
| Recurrence | |
| No | 305 (88.7) |
| Yes | 39 (11.3) |
| Metastasis | |
| No | 241 (70.1) |
| Yes | 103 (29.9) |
| DFS (days) | 1,399.1 (6–45,187) |
SqCC, squamous cell carcinoma; NOS, not otherwise specified; OP, operation; CTx, chemotherapy; RTx, radiotherapy; PD-L1, programmed cell death ligand-1; DFS, disease-free survival.
Among resection cases (n=154), tumor size averaged 2.8 cm (0.6–10.2 cm) (Table 2). The adenocarcinoma subtypes were acinar in 71 cases (60.7%), micropapillary in three cases (2.6%), papillary in seven cases (6.0%), solid in 16 cases (13.7%), lepidic in 13 cases (11.1%), adenocarcinoma in situ (AIS), and minimally invasive adenocarcinoma (MIA) in seven cases (6.0%).
Table 2
| Clinicopathologic factors | Value, n (%) or mean (range) |
|---|---|
| Size (cm) | 2.75 (0.6–10.2) |
| Adenocarcinoma subtype (n=117) | |
| Acinar | 71 (60.7) |
| Micropapillary | 3 (2.6) |
| Papillary | 7 (6.0) |
| Solid | 16 (13.7) |
| Lepidic | 13 (11.1) |
| AIS, MIA | 7 (6.0) |
| pT stage (TNM8) | |
| pTis | 2 (1.3) |
| pT1 | 38 (24.7) |
| pT2 | 97 (63.0) |
| pT3 | 12 (7.8) |
| pT4 | 5 (3.2) |
| Visceral pleural invasion | |
| No | 51 (33.1) |
| Yes | 103 (66.9) |
| Lymphatic invasion | |
| No | 137 (89.0) |
| Yes | 17 (11.0) |
| Venous invasion | |
| No | 154 (100.0) |
| Yes | 0 (0.0) |
| Perineural invasion | |
| No | 153 (99.4) |
| Yes | 1 (0.6) |
AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; TNM8, tumor-node-metastasis staging system 8th edition.
SP263: results and correlation with clinicopathologic features
Using the SP263 antibody, all cases were classified as follows: negative, 155 cases (45.1%); low expression, 131 cases (38.1%); and high expression, 58 cases (16.9%). Correlations between PD-L1 expression and clinicopathological features in all cases revealed statistically significant differences in age (P=0.03), sex (P=0.001), tumor type (P<0.001), and specimen type (P=0.03) among the negative, low expression, and high expression groups (Table 3). There were no significant differences in recurrence (P=0.16) or metastasis (P=0.40).
Table 3
| Clinicopathologic factors | N | SP263 | SP142 TC | SP142 IC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n=155) | Low+ (n=131) | High+ (n=58) | P | Negative (n=281) | Low+ (n=51) | High+ (n=12) | P | Negative (n=216) | Low+ (n=128) | High+ (n=0) | P | ||||
| Age (years) | 0.033 | 0.319 | >0.99 | ||||||||||||
| <70 | 140 | 73 (52.1) | 42 (30.0) | 25 (7.3) | 110 (78.6) | 23 (16.4) | 7 (5.0) | 88 (62.9) | 52 (37.1) | 0 (0) | |||||
| ≥70 | 204 | 82 (40.2) | 89 (43.6) | 33 (16.2) | 171 (83.8) | 28 (13.7) | 5 (2.5) | 128 (62.7) | 76 (37.3) | 0 (0) | |||||
| Sex | 0.001 | 0.105 | 0.559 | ||||||||||||
| Male | 223 | 84 (37.7) | 95 (42.6) | 44 (19.7) | 175 (78.5) | 38 (17.0) | 10 (4.5) | 137 (61.4) | 86 (38.6) | 0 (0) | |||||
| Female | 121 | 71 (58.7) | 36 (29.8) | 14 (11.6) | 106 (87.6) | 13 (10.7) | 2 (1.7) | 79 (65.3) | 42 (34.7) | 0 (0) | |||||
| Tumor type | <0.001 | 0.001 | 0.333 | ||||||||||||
| Adenocarcinoma | 247 | 132 (53.4) | 82 (33.2) | 33 (13.4) | 212 (85.8) | 25 (10.1) | 10 (4.0) | 161 (65.2) | 86 (34.8) | 0 (0) | |||||
| SqCC | 83 | 17 (20.5) | 44 (53.0) | 22 (26.5) | 56 (67.5) | 25 (30.1) | 2 (2.4) | 47 (56.6) | 36 (43.4) | 0 (0) | |||||
| NOS | 14 | 6 (42.9) | 5 (35.7) | 3 (21.4) | 13 (92.9) | 1 (7.1) | 0 (0) | 8 (57.1) | 6 (42.9) | 0 (0) | |||||
| Specimen type | 0.037 | 0.734 | 0.033 | ||||||||||||
| Biopsy | 190 | 74 (38.9) | 82 (43.2) | 34 (17.9) | 155 (81.6) | 27 (14.2) | 8 (4.2) | 129 (67.9) | 61 (32.1) | 0 (0) | |||||
| Resection | 154 | 81 (52.6) | 49 (31.8) | 24 (15.6) | 126 (81.8) | 24 (15.6) | 4 (2.6) | 87 (56.5) | 67 (43.5) | 0 (0) | |||||
| Recurrence | 0.162 | 0.577 | 0.385 | ||||||||||||
| No | 305 | 142 (46.6) | 115 (37.7) | 48 (15.7) | 248 (81.3) | 47 (15.4) | 10 (3.3) | 194 (63.6) | 111 (36.4) | 0 (0) | |||||
| Yes | 39 | 13 (33.3) | 16 (41.0) | 10 (25.6) | 33 (84.6) | 4 (10.3) | 2 (5.1) | 22 (56.4) | 17 (43.6) | 0 (0) | |||||
| Metastasis | 0.406 | 0.246 | 0.015 | ||||||||||||
| No | 241 | 114 (47.3) | 89 (36.9) | 38 (15.8) | 197 (81.7) | 38 (15.8) | 6 (2.5) | 141 (58.5) | 100 (41.5) | 0 (0) | |||||
| Yes | 103 | 41 (39.8) | 42 (40.8) | 20 (19.4) | 84 (81.6) | 13 (12.6) | 6 (5.8) | 75 (72.8) | 28 (27.2) | 0 (0) | |||||
Data are presented as n (%). PD-L1, programmed cell death ligand-1; TC, tumor cell; IC, immune cell; SqCC, squamous cell carcinoma; NOS, not otherwise specified.
The OR between PD-L1 expression and the clinicopathological features in all cases revealed that the PD-L1 expression rate was significantly higher in older patients than in younger patients (OR, 1.62; 95% CI: 1.05–2.50; P=0.02), male sex than female sex (OR, 2.35; 95% CI: 1.49–3.69; P<0.001), SqCC than in adenocarcinoma (OR, 4.45; 95% CI: 2.47–8.02; P<0.001), and biopsy than in resection samples (OR, 0.57; 95% CI: 0.37–0.88; P=0.01) in univariate analysis (Table 4). There was no significant association with recurrence (OR, 1.74; 95% CI: 0.86–3.51; P=0.12) or metastasis (OR, 1.35; 95% CI: 0.85–2.16; P=0.20). In multivariate analysis, the PD-L1 expression rate was significantly higher in males than in females (OR, 1.68; 95% CI: 1.03–2.73; P=0.03) and in SqCC than in adenocarcinoma (OR, 3.47; 95% CI: 1.87–6.44; P<0.001). There was no significant association with the specimen types (OR, 0.66; 95% CI: 0.42–1.05; P=0.08).
Table 4
| Clinicopathologic factors | SP263 (cutoff ≥1%) | SP142 TC (cutoff ≥1%) | SP142 IC (cutoff ≥1%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||||||
| Age (years) (<70avs. ≥70) |
1.621 (1.050–2.502) |
0.029 | 0.708 (0.408–1.226) |
0.217 | 0.478 (0.258–0.884) |
0.019 | 1.005 (0.644–1.568) |
0.983 | |||||||||
| Sex (femaleavs. male) |
2.350 (1.495–3.693) |
<0.001 | 1.685 (1.039–2.732 |
0.034 | 1.938 (1.034–3.632) |
0.039 | 1.181 (0.744–1.873) |
0.480 | |||||||||
| Tumor type | |||||||||||||||||
| Adenocarcinoma | Ref | <0.001 | Ref | <0.001 | Ref | 0.001 | Ref | <0.001 | Ref | 0.344 | |||||||
| SqCC | 4.456 (2.473–8.029) |
<0.001 | 3.475 (1.874–6.444) |
<0.001 | 2.920 (1.632–5.227) |
<0.001 | 3.808 (2.008–7.221) |
<0.001 | 1.434 (0.864–2.381) |
0.163 | |||||||
| NOS | 1.530 (0.516–4.541) |
0.443 | 1.063 (0.345–3.279) |
0.915 | 0.466 (0.059–3.675) |
0.469 | 0.541 (0.068–4.320) |
0.562 | 1.404 (0.472–4.718) |
0.542 | |||||||
| Specimen type (biopsyavs. resection) |
0.575 (0.374–0.884) |
0.012 | 0.668 (0.421–1.058) |
0.086 | 0.984 (0.568–1.705) |
0.955 | 1.629 (1.048–2.531) |
0.030 | |||||||||
| Recurrence (noavs. yes) |
1.742 (0.863–3.518) |
0.122 | 0.791 (0.316–1.978) |
0.616 | 1.351 (0.688–2.651) |
0.383 | |||||||||||
| Metastasis (noavs. yes) |
1.357 (0.850–2.169) |
0.201 | 1.013 (0.558–1.837) |
0.967 | 0.526 (0.318–0.871) |
0.013 | 0.526 (0.318–0.871) |
0.013 | |||||||||
a, reference. PD-L1, programmed cell death ligand-1; TC, tumor cell; IC, immune cell; OR, odds ratio; CI, confidence interval; Ref, reference; SqCC, squamous cell carcinoma; NOS, not otherwise specified.
OR between PD-L1 expression and the clinicopathological features in resection cases revealed that PD-L1 expression rate was significantly higher in males than in females (OR, 3.21; 95% CI: 1.62–6.35; P=0.001), SqCC than in adenocarcinoma (OR, 6.55; 95% CI: 2.33–18.43; P<0.001), cases with recurrence than in those without (OR, 2.42; 95% CI: 1.00–5.85; P=0.04), size ≥4 cm than in <4 cm (OR, 3.96; 95% CI: 1.15–13.56; P=0.02), and cases with solid components than in those without (OR, 5.31; 95% CI: 1.42–19.84; P=0.01) in univariate analysis (Table 5). There was no significant association with age (OR, 1.495; 95% CI: 0.79–2.82; P=0.21), pathological T stage (OR, 1.66; 95% CI: 0.77–3.55; P=0.19), visceral pleural invasion (OR, 0.53; 95% CI: 0.14–1.95; P=0.34), lymphatic invasion (OR, 2.16; 95% CI: 0.61–7.63; P=0.23), or perineural invasion (OR, <0.001; P>0.99). In the multivariate analysis, there was no significant association with the solid components (OR, 8.33; 95% CI: 0.88–78.31; P=0.06).
Table 5
| Clinicopathologic factors | SP263 (cutoff ≥1%) | SP142 TC (cutoff ≥1%) | SP142 IC (cutoff ≥1%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||||||
| Age (years) (<70avs. ≥70) |
1.495 (0.790–2.829) |
0.217 | 1.049 (0.461–2.385) |
0.909 | 1.060 (0.559–2.009) |
0.859 | |||||||||||
| Sex (femaleavs. male) |
3.216 (1.628–6.354) |
0.001 | 2.933 (1.113–7.728) |
0.029 | 1.243 (0.647–2.387) |
0.513 | |||||||||||
| Tumor type | |||||||||||||||||
| Adenocarcinoma | Ref | <0.001 | Ref | <0.001 | Ref | 0.337 | |||||||||||
| SqCC | 6.557 (2.332–18.436) |
<0.001 | 8.692 (3.405–22.189) |
<0.001 | 1.504 (0.654–3.459) |
0.337 | |||||||||||
| Recurrence (noavs. yes) |
2.429 (1.007–5.856) |
0.048 | 0.537 (0.149–1.933) |
0.342 | 1.138 (0.488–2.653) |
0.765 | |||||||||||
| Metastasis (noavs. yes) |
0.361 (0.037–3.551) |
0.382 | <0.001# | 0.999 | 1.308 (0.179–9.532) |
0.791 | |||||||||||
| Size (<4avs. ≥4 cm) |
3.960 (1.156–13.565) |
0.028 | 1.708 (0.357–8.164) |
0.503 | 1.471 (0.462–4.680) |
0.514 | |||||||||||
| Stage (pTis & T1 & T2aavs. T2b & T3 & T4) | 1.660 (0.776–3.553) |
0.191 | 1.822 (0.739–4.494) |
0.193 | 1.303 (0.612–2.776) |
0.492 | |||||||||||
| Solid (noavs. yes) | 5.318 (1.425–19.848 |
0.013 | 8.333 (0.887–78.311) |
0.064 | 1.708 (0.488–5.974) |
0.402 | 9.500 (1.038–86.968) |
0.046 | 2.317 (0.780–6.883) |
0.130 | |||||||
| Visceral pleural invasion (noavs. yes) | 0.539 (0.149–1.954) |
0.347 | 0.527 (0.127–2.192) |
0.379 | 0.647 (0.185–2.255) |
0.494 | |||||||||||
| Lymphatic invasion (noavs. yes) | 2.163 (0.613–7.638) |
0.231 | 6.286 (1.775–22.256) |
0.004 | 1.845 (0.549–6.205) |
0.322 | |||||||||||
| Perineural invasion (noavs. yes) | <0.001# | >0.99 | <0.001# | >0.99 | <0.001# | >0.99 | |||||||||||
a, reference; #, statistically insignificant results. PD-L1, programmed cell death ligand-1; TC, tumor cell; IC, immune cell; OR, odds ratio; CI, confidence interval; Ref, reference; SqCC, squamous cell carcinoma.
Correlations between PD-L1 expression and adenocarcinoma subtype in resection cases were identified at both 1% and 50% cut-off values (P=0.005) (Table 6). The OR between PD-L1 expression and the adenocarcinoma subtype in resection cases revealed that the PD-L1 expression rate was significantly higher in the solid subtype than in the acinar subtype (OR, 4.60; 95% CI: 1.35–15.72; P=0.01) (Table 7). There were no significant associations with micropapillary (OR, 3.07; 95% CI: 0.26–35.49; P=0.36), papillary (OR, 0.61; 95% CI: 0.11–3.38; P=0.57), lepidic (OR, 0.27; 95% CI: 0.05–1.35; P=0.11), or AIS, MIA (OR, 0.61; 95% CI: 0.11–3.38; P=0.57). There was no significant difference between PD-L1 expression and DFS in resection cases using the 1% cut-off (P=0.25) and 50% cut-off (P=0.14) values (Table 8).
Table 6
| Adenocarcinoma subtype | SP263 | SP142 TC | SP142 IC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n=69) | Low+ (n=37) | High+ (n=11) | P | Negative (n=103) | Low+ (n=12) | High+ (n=2) | P | Negative (n=65) | Low+ (n=52) | High+ (n=0) | P | |||
| Acinar (N=71) | 43 (60.6) | 25 (35.2) | 3 (4.2) | 0.005 | 63 (88.7) | 8 (11.3) | 0 (0) | 0.131 | 43 (60.6) | 28 (39.4) | 0 (0) | 0.207 | ||
| Micropapillary (N=3) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 3 (100.0) | 0 (0) | 0 (0) | 1 (33.3) | 2 (66.7) | 0 (0) | |||||
| Papillary (N=7) | 5 (71.4) | 1 (14.3) | 1 (14.3) | 5 (71.4) | 2 (28.6) | 0 (0) | 1 (14.3) | 6 (85.7) | 0 (0) | |||||
| Solid (N=16) | 4 (25.0) | 6 (37.5) | 6 (37.5) | 12 (75.0) | 2 (12.5) | 2 (12.5) | 8 (50.0) | 8 (50.0) | 0 (0) | |||||
| Lepidic (N=13) | 11 (84.6) | 2 (15.4) | 0 (0) | 13 (100.0) | 0 (0) | 0 (0) | 7 (53.8) | 6 (46.2) | 0 (0) | |||||
| AIS, MIA (N=7) | 5 (71.4) | 2 (28.6) | 0 (0) | 7 (100.0) | 0 (0) | 0 (0) | 5 (71.4) | 2 (28.6) | 0 (0) | |||||
Data are presented as n (%). PD-L1, programmed cell death ligand-1; TC, tumor cell; IC, immune cell; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma.
Table 7
| Adenocarcinoma subtype | SP263 (cutoff ≥1%) | SP142 TC (cutoff ≥1%) | SP142 IC (cutoff ≥1%) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| Acinar | Reference | 0.049 | Reference | 0.717 | Reference | 0.345 | ||
| Micropapillary | 3.071 (0.266–35.493) | 0.369 | 0# | 0.999 | 3.071 (0.266–35.493) | 0.369 | ||
| Papillary | 0.614 (0.111–3.388) | 0.576 | 3.150 (0.522–19.004) | 0.211 | 9.214 (1.052–80.689) | 0.045 | ||
| Solid | 4.607 (1.350–15.724) | 0.015 | 2.625 (0.681–10.123) | 0.161 | 1.536 (0.517–4.565) | 0.440 | ||
| Lepidic | 0.279 (0.058–1.356) | 0.114 | 0# | 0.999 | 1.316 (0.401–4.326) | 0.651 | ||
| AIS, MIA | 0.614 (0.111–3.388) | 0.576 | 0# | 0.999 | 0.614 (0.111–3.388) | 0.576 | ||
#, statistically insignificant results. PD-L1, programmed cell death ligand-1; TC, tumor cell; IC, immune cell; OR, odds ratio; CI, confidence interval; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma.
Table 8
| SP263 | SP 142 TC | SP142 IC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1% | ≥1% | P | <50% | ≥50% | P | <1% | ≥1% | P | <50% | ≥50% | P | <1% | ≥1% | P | |||
| DFS (day) | 1,057.50 | 1,679.21 | 0.255 | 1,070.81 | 3,017.81 | 0.141 | 1,214.60 | 2,221.94 | 0.322 | 1,422.68 | 746.17 | 0.665 | 1,519.27 | 1,196.27 | 0.586 | ||
DFS, disease-free survival; PD-L1, programmed cell death ligand-1; TC, tumor cell; IC, immune cell.
SP142 TC: results and correlation with clinicopathological features
SP142 TC expression was classified as follows: negative, 281 cases (81.7%); low, 51 cases (14.8%); and high, 12 cases (3.5%). Correlations between PD-L1 expression and the clinicopathological features in all cases revealed a statistically significant difference in tumor type (P=0.001) among the negative, low expression, and high expression groups. There were no significant differences in age (P=0.31), sex (P=0.10), specimen type (P=0.73), recurrence (P=0.57), or presence of metastasis (P=0.24).
OR between PD-L1 expression and the clinicopathological features in all cases revealed that the PD-L1 expression rate was significantly higher in males than in females (OR, 1.93; 95% CI: 1.03–3.63; P=0.03) and in SqCC than in adenocarcinoma (OR, 2.92; 95% CI: 1.63–5.22; P<0.001) in univariate analysis. There were no significant associations with age (OR, 0.70; 95% CI: 0.40–1.22; P=0.21), specimen type (OR, 0.98; 95% CI: 0.56–1.70; P=0.95), recurrence (OR, 0.79; 95% CI: 0.31–1.97; P=0.61), and metastasis (OR, 1.01; 95% CI: 0.55–1.83; P=0.96) in the univariate analysis. In multivariate analysis, the PD-L1 expression rate was significantly higher in younger patients than in older patients (OR, 0.47; 95% CI: 0.25–0.88; P=0.019) and in SqCC than in adenocarcinoma (OR, 3.80; 95% CI: 2.00–7.22; P<0.001).
OR between PD-L1 expression and the clinicopathological features in resection cases revealed that the PD-L1 expression rate was significantly higher in males than in females (OR, 2.93; 95% CI: 1.11–7.72; P=0.02), in SqCC than in adenocarcinoma (OR, 8.69; 95% CI: 3.40–22.18; P<0.001), and in cases with lymphatic invasion than in those without (OR, 6.28; 95% CI: 1.77–22.25; P=0.004) in univariate analysis. There was no significant association with age (OR, 1.04; 95% CI: 0.46–2.38; P=0.90), recurrence (OR, 0.53; 95% CI: 0.14–1.93; P=0.34), metastasis (OR, <0.001; P>0.99), tumor size (OR, 1.70; 95% CI: 0.35–8.16; P=0.50), pathologic T stage (OR, 1.82; 95% CI: 0.73–4.49; P=0.19), solid component (OR, 1.70; 95% CI: 0.48–5.97; P=0.40), visceral pleural invasion (OR, 0.52; 95% CI: 0.12–2.19; P=0.37), and perineural invasion (OR, <0.001; P>0.99). In the multivariate analysis, the PD-L1 expression rate was significantly higher in cases with a solid component than in those without (OR, 9.50; 95% CI: 1.03–86.96; P=0.04).
A correlation between PD-L1 expression and the adenocarcinoma subtype in resection cases was not identified. OR between PD-L1 expression and adenocarcinoma subtype in resection cases revealed no significant associations between micropapillary (P>0.99), papillary (OR, 3.15; 95% CI: 0.52–19.00; P=0.21), solid (OR, 2.62; 95% CI: 0.68–10.12; P=0.16), lepidic (P>0.99), AIS, MIA (P>0.99). There was no significant difference between PD-L1 expression and DFS in resection cases using the 1% cut-off (P=0.32) and 50% cut-off values (P=0.65).
SP142 IC: results and correlation with clinicopathological features
SP142 IC expression was classified as follows: negative, 216 cases (62.8%), and low expression, 128 cases (37.2%). There were no IC-high SP142 IC high-expression case. Correlations between PD-L1 expression and the clinicopathological features in all cases revealed statistically significant differences in specimen type (P=0.03) and metastasis (P=0.01) between the negative and low-expression groups. There were no significant differences in age (P>0.99), sex (P=0.55), tumor type (P=0.33), or recurrence (P=0.38).
OR between PD-L1 expression and the clinicopathological features in all cases revealed that the PD-L1 expression rate was significantly higher in resection than in biopsy (OR, 1.629; 95% CI: 1.04–2.53; P=0.03) and in cases without metastasis than in those with metastasis (OR, 0.52; 95% CI: 0.31–0.87; P=0.01) in univariate analysis. There were no significant associations with age (OR, 1.00; 95% CI: 0.64–1.56; P=0.98), sex (OR, 1.18; 95% CI: 0.74–1.87; P=0.48), SqCC (OR, 1.43; 95% CI: 0.86–2.38; P=0.16), or recurrence (OR, 1.35; 95% CI: 0.68–2.65; P=0.38). In multivariate analysis, the PD-L1 expression rate was significantly higher in patients without metastasis than in those with metastasis (OR, 0.52; 95% CI: 0.31–0.87; P=0.01).
OR between PD-L1 expression and the clinicopathological features in resection cases revealed that there was no significant association with age (OR, 1.06; 95% CI: 0.55–2.00; P=0.85), sex (OR, 1.24; 95% CI: 0.64–2.38; P=0.51), SqCC (OR, 1.50; 95% CI: 0.65–3.45; P=0.33), recurrence (OR, 1.13; 95% CI: 0.48–2.65; P=0.76), metastasis (OR, 1.30; 95% CI: 0.17–9.53; P=0.79), size (OR, 1.47; 95% CI: 0.46–4.68; P=0.51), pathologic T stage (OR, 1.30; 95% CI: 0.61–2.77; P=0.49), solid component (OR, 2.31; 95% CI: 0.78–6.88; P=0.13), visceral pleural invasion (OR, 0.64; 95% CI: 0.18–2.25; P=0.49), lymphatic invasion (OR, 1.84; 95% CI: 0.54–6.20; P=0.32), and perineural invasion (P>0.99).
A correlation between PD-L1 expression and the adenocarcinoma subtype in resection cases was not identified. The OR between PD-L1 expression and the adenocarcinoma subtype in resection cases revealed that the PD-L1 expression rate was significantly higher in the papillary subtype than in the acinar subtype (OR, 9.21; 95% CI: 1.05–80.68; P=0.04). There were no statistically significant associations with micropapillary (OR, 3.07; 95% CI: 0.26–35.49; P=0.36), solid (OR, 1.53; 95% CI: 0.51–4.56; P=0.44), lepidic (OR, 1.31; 95% CI: 0.40–4.32; P=0.65), or AIS, MIA (OR, 0.61; 95% CI: 0.11–3.38; P=0.57). There was no significant difference between PD-L1 expression and DFS in the resection cases using the 1% cutoff value (P=0.58).
Combined SP142 TC and/or IC: results and correlation with clinicopathological features
Even though we evaluated the TC and IC separately, we tried to combine the two data values into one result (Table S1). In the combined results of SP142 TC or SP142 IC, all cases were classified as follows: negative, 192 cases (55.8%) and positive, 152 cases (44.2%). In the combined results of SP142 TC and SP142 IC, all cases were classified as follows: negative, 305 cases (88.7%) and positive, 39 cases (11.3%). In the combined results of SP142TC or SP142IC expression, there were significant differences in tumor type (P=0.002) and metastasis (P=0.04). There were no significant differences in age (P=0.74), sex (P=0.17), specimen type (P=0.06), and recurrence (P=0.60). The combined results for SP142TC and SP142IC expression showed significant differences with respect to age (P=0.03). There were no significant differences in sex (P=0.21), tumor type (P=0.21), specimen type (P=0.86), recurrence (P>0.99), or metastasis (P=0.58).
OR between PD-L1 expression (SP142 TC or SP142 IC expression, and both SP142 TC and SP142 IC expression, cutoff ≥1%) and the clinicopathological features are summarized in Table S2. In the combined results of SP142TC or SP142IC expression, the PD-L1 expression rate was significantly higher in SqCC than in adenocarcinoma (OR, 2.42; 95% CI: 1.45–4.03; P=0.001) and in cases without metastasis than in those with metastasis (OR, 0.61; 95% CI: 0.38–0.98; P=0.04) in univariate analysis. There were no significant associations with age (P=0.69), sex (P=0.14), specimen type (P=0.05), or recurrence (P=0.54). In the multivariate analysis, the PD-L1 expression rate was significantly higher in SqCC than in adenocarcinoma (OR, 2.79; 95% CI: 1.64–4.74; P<0.001) and in resection than in biopsy cases (OR, 1.89; 95% CI: 1.19–2.99; P=0.007). In the combined results of SP142TC and SP142IC expression, the PD-L1 expression rate was significantly higher in younger patients than in older patients (OR, 0.48; 95% CI: 0.24–0.95; P=0.03) in the univariate analysis. There were no significant associations with sex (P=0.19), tumor type (P=0.46), specimen type (P=0.85), recurrence (P=0.82), or metastasis (P=0.53). In multivariate analysis, the PD-L1 expression rate was significantly higher in younger patients than in older patients (OR, 0.48; 95% CI: 0.24–0.95; P=0.03).
Concordance rate of PD-L1 expression between SP263 and SP142
Using Cohen’s Kappa Coefficient, the concordance rate between SP263 and SP142 TC was 0.234. Also taking ICs into account, concordance rate between SP263 and combined results of SP142 TC or IC was 0.247.
Discussion
Immunotherapy has shown remarkable therapeutic effects as a second-line therapy for various types of advanced cancers, including primary lung cancer. Thus, investigating PD-L1 expression status is crucial for predicting the therapeutic response to anti-PD-L1 agents. Currently, immunohistochemical staining for PD-L1 has become a standard test for evaluating PD-L1 expression, and it is the only validated assay for application of anti-PD-L1 drugs. However, studies have indicated a poor correlation between the immunohistochemical assay and the actual response (4,13-17). Furthermore, each cutoff set for the application of anti-PD-L1 agents has failed to separate responders from non-responders (16,19). These features render PD-L1 expression unreliable in predicting treatment response to anti-PD-L1 treatment, as well as prognosis.
Under these circumstances, many researchers have focused on the prognostic effects and clinicopathological factors associated with PD-L1 expression (16,18,21-31) (Table 9). Many studies have reported that PD-L1 expression is associated with an unfavorable prognosis, including shorter overall survival and relapse-free survival (16,18,21-27). Others have reported that PD-L1 expression is associated with a favorable prognosis, including longer overall survival, relapse-free survival, and median survival (28-31). Regarding clinicopathological factors, PD-L1 expression has been associated with male sex (23-25), age (24,28), smoking (23-25), tumor type and histological subtype (16,18,23,24,26,27), tumor size (16), tumor grade (16,22,23,28,31), tumor stage (22,23,26), vascular invasion (24,31), and lymph node metastasis (23). Some reports also revealed conflicting results that high PD-L1 expression was associated with both favorable prognosis and poor prognostic factors, including high-grade or advanced pathologic features such as higher tumor grade and vascular invasion (28,31). Many studies presumed that PD-L1 expression might serve as an indicator of an anti-tumoral host immune response rather than signaling tumor immune evasion, and that the overall balance of the anti-tumor response by the host and immune suppression by the tumor might be related. However, there is insufficient evidence to support this hypothesis. These variable results might be due to several factors, including different anti-PD-L1 antibodies and cutoff values used (24), or other clinicopathological factors that are involved.
Table 9
| Authors | Case No. | Clones | Association of prognosis |
Associated clinicopathologic factors |
|---|---|---|---|---|
| Cooper et al. | 681 | 22C3 | Favorable | Younger age, higher tumor grade, longer OS |
| Velcheti et al. | 544 | ab58810, MIH1, 29E.2A3, 5H1 |
Favorable | Local lymphocytic infiltration, longer OS |
| Yang et al. | 163 | N/A | Favorable | Higher tumor grade, vascular invasion, longer RFS |
| Shah et al. | 166 | 22C3 | Favorable | Longer OS and MS |
| Montero et al. | 482 | 28-8, 22C3, SP263, SP142 |
Unfavorable | Tumor type, tumor size, grading, shorter OS |
| Azuma et al. | 164 | N/A | Unfavorable | EGFR mutation, shorter OS |
| Chen et al. | 120 | 236A/E7 | Unfavorable | Higher tumor grade, advanced stage, shorter OS |
| Mu et al. | 109 | N/A | Unfavorable | Adenocarcinoma tumor type, shorter OS |
| Zhang et al. | 143 | SAB2900365 | Unfavorable | Solid predominant subtype of adenocarcinoma, advanced stage, shorter OS |
| Wu et al. | 133 | SP263 | Unfavorable | Male sex, smoking, shorter RFS and OS |
| Cha et al. | 323 | SP142 | Unfavorable | Smoking, solid predominant type, p53 aberrant expression, PD-L1 expression in tumor-infiltrating immune cells, shorter RFS and OS |
| Okita et al. | 91 | SP142 | Unfavorable | Male sex, smoking, squamous cell carcinoma tumor type, histologic grade, lymph node metastasis, pathological stage, shorter RFS and OS |
| Takada et al. | 499 | SP142 | Unfavorable | Male sex, smoking, older age, vascular invasion, squamous cell carcinoma, EGFR-wildtype, shorter OS |
PD-L1, programmed cell death ligand-1; OS, overall survival; N/A, not available; RFS, relapse-free survival; MS, median survival; EGFR, epidermal growth factor receptor.
In this study, we identified several significant clinicopathological factors associated with PD-L1 expression. First, SqCC was associated with high PD-L1 expression in SP263, SP142 TC, and combined SP142 positive TC or IC cells, similar to those of previous studies (16,18,23,24). It has been postulated that SqCC is associated with cigarette smoking history, and previous studies have indicated that smoking is associated with high PD-L1 expression (23-25,27). Considering that smoking-associated lung cancers had more somatic gene alterations than never smokers in previous studies, SqCC might be better regarded as a phenotype of larger tumor mutation burden, which is related to poor prognostic factors without treatment (24,34,35). Although we did not investigate smoking history in this study, these two clinicopathological factors appeared to be closely associated with high PD-L1 expression.
In particular, we focused on the finding that the presence of a solid component of adenocarcinoma was associated with high PD-L1 expression in SP263 and SP142 positive cells. Pathologically, the presence of a solid component is associated with a higher tumor grade and poor tumor differentiation, and the solid-predominant subtype is associated with poor prognosis (36-39). In several studies, high PD-L1 expression was associated with a higher tumor grade, including solid components and poor tumor differentiation (16,22,23,26-28,31). In addition, Miyazawa et al. reported that even a small amount of solid component (≥5%) was associated with PD-L1 positivity (40). In our study, not only the solid-predominant subtype of adenocarcinoma, but also cases with small amounts of solid components were associated with high PD-L1 expression, which is consistent with the previous studies. It has been suggested that PD-L1 expression may be significantly associated with poor pathological prognostic components; however, this relationship is not well known.
Another important finding was that lymphatic invasion was associated with high PD-L1 expression in SP142 TC. Lymphovascular invasion is a poor prognostic factor. Higgins et al. reported that lymphovascular invasion is associated with adverse prognostic factors, including poor overall survival and lymph node metastasis (41). We observed high PD-L1 expression associated with lymphovascular invasion, consistent with previous findings (23,24,31). This finding supports the assumption that high PD-L1 expression is associated with an unfavorable prognosis.
Consistent with the association between high PD-L1 expression and adverse clinicopathological factors, recurrence was associated with high PD-L1 expression in SP263. Kojima et al. reported an association between PD-L1 and recurrence in NSCLC, and demonstrated that the risk of postoperative recurrence increased with an increase in PD-L1 expression using the 22C3 clone (42). They also revealed that high PD-L1 expression in patients who underwent resection was an independent risk factor for recurrence. Their report supports the results of our study, suggesting that PD-L1 expression is associated with adverse prognostic factors. Considering that the interpretation of the SP263 assay only calculates TCs and not tumor-infiltrating lymphocytes, this association might be the result of an immune evasion mechanism by TCs expressing PD-L1, which renders TCs more vulnerable to recurrence.
PD-L1 expression is known to be associated with metastasis to other organs, including the esophagus and oral cavity (43,44). However, despite the association between PD-L1 expression and several adverse prognostic factors, the association between PD-L1 expression and metastasis in primary lung cancer is unknown. As PD-L1 expression has been assumed to be associated with adverse clinicopathological factors, it has also been presumed to be associated with metastasis in lung cancer. In previous studies, PD-L1 expression in NSCLC was revealed to be associated with WIP and β-catenin signaling pathways, which have been known to be involved in cancer invasion and metastasis (45,46). However, the overall or net effect of high PD-L1 expression on NSCLC metastasis remains unclear. In this study, cases without metastasis were associated with high PD-L1 expression in SP142 IC. To our knowledge, this is the first study to reveal an inverse relationship between PD-L1 expression and metastasis in NSCLC. This paradoxical result might be derived from differences in interpreting cells, considering that only tumor-infiltrating ICs were calculated for PD-L1 expression in the SP142 IC. Tumor-infiltrating ICs, recognized as antitumor host immune responses, are proposed to exert protective effects against metastasis, resulting in this association. Previous studies have revealed that high levels of FOXP3+ T cells, a subset of tumor-infiltrating lymphocytes, are associated with unfavorable prognostic factors, including shorter overall and recurrence-free survival (47,48). In contrast to previous studies that focused on PD-L1 expression in TCs, we emphasized the role of PD-L1 expression in tumor-infiltrating ICs during metastasis. However, owing to the small sample size of the SP142 IC group, further studies are needed to verify this association.
The association between age and PD-L1 expression is somewhat unclear. Older age was associated with high PD-L1 expression in SP263 positive TCs, whereas younger age was associated with high PD-L1 expression in SP142 TC and combined SP142TC and IC. Cooper et al. (28) reported an association between younger age and PD-L1 expression of 22C3. In contrast Takada et al. (24) revealed an association between older age and PD-L1 expression in SP142, which contradicts the results of our study. These conflicting results may be due to several factors, including the use of different PD-L1 clones, variations in assay interpretation methods, and different age cutoffs. This emphasizes the need to specify the PD-L1 clones and interpretation methods when reporting PD-L1 expression, as the associated clinical factors may vary greatly among them.
Our study found a consistent association between male sex and high PD-L1 expression in SP263 and SP142 TC, similar to those of previous studies (23-25). This suggest that male patients should be more considered for PD-L1 assay and anti-PD-L1 treatment. However, we could not observe statistically significant difference of DFS in both male and female patients expressing PD-L1 expression, and further studies are needed.
Meanwhile, different results between SP263 and SP142 were seen, which were compatible with the results of Blueprint Phase 2 (BP2) study (49). In our study, concordance rate between SP263 and SP142 TC was 0.234, and concordance rate between SP263 and combined results of SP142 TC or IC was 0.247, both of them revealing relatively poor degree of agreement as analytic methods. Our results support the conclusion in BP2 study that SP142 seems to lack interchangeability with SP263, and it might be related to complex and nonintuitive IC scoring methods in SP142. In addition, comparing the relationship between clinicopathological factors and PD-L1 expression between SP263 and SP142, it is evident that SP263 is more sensitive and reveals more correlated factors than SP142. Hence, it is presumed that SP263 would be more eligible for daily practice than SP142, until more simplified and reproducible scoring methods are established for SP142. However, they both failed to reveal correlation with DFS. Although PD-L1 positivity seems to be related to adverse prognostic factors, it also enables more treatment options, namely anti-PD-L1 agents. Indeed, some of the patients with PD-L1 expression received anti-PD-L1 therapies, and they might have influenced on the results.
This study had several limitations. First, this was a retrospective study conducted at a single institution, which might have been prone to several biases, including selection bias and bias from variable treatments. Second, in the resection cases, only representative sections from the tumors were used to evaluate PD-L1 positivity. Owing to tumor heterogeneity, the results might be inconsistent with those from the immunostaining of whole-section slides. Third, biopsy specimens are much smaller than resection specimens, and although the former have less pathological information than the latter, they were analyzed together in comparison with some clinicopathological parameters. Fourth, we tried to include smoking history in clinicopathologic factors as correlation of smoking history and PD-L1 expression was reported in some previous studies. However, collecting the clinical data, we found out that smoking history was inappropriately investigated on the medical records of the patients, revealing conflicting comments within each of them. Thus, we decided not to include smoking history for evaluation in this current study, but in the future studies. Fifth, we could not observe statistically significant difference among subtypes of resected adenocarcinoma cases, probably due to small number in each category. Finally, mononucleated ICs are composed of CD3+ T and CD20+ B cells, but they cannot be morphologically discriminated via H&E stain. As they are heterogeneous population of variable inflammatory cells, their discrimination could have facilitated further investigation. However, in the setting of observational retrospective study, immunotyping was not implemented.
Conclusions
In conclusion, PD-L1 expression in NSCLC is associated with adverse clinicopathological features and recurrence. Therefore, it could be utilized to predict poor prognosis. Furthermore, the high PD-L1 expression of SP142 in tumor-infiltrating ICs could be a potential marker for low metastasis. These findings could help in further establishing criteria for identifying responders and non-responders to anti-PD-L1 therapy and guide treatment approaches.
Acknowledgments
Funding: This work was supported by a
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-812/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-812/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-812/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-812/coif). Y.J.L. reports that this work was supported by a Korea University Grant (No. K2110591) and SK Bioscience Co., Ltd. (No. Q2208721). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Korea University Anam Hospital (IRB No. 2022AN0558). The study was conducted in retrospective way, and informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Es-timates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- van den Bulk J, Verdegaal EM, de Miranda NF. Cancer immunotherapy: broadening the scope of targetable tumours. Open Biol 2018;8:180037. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brody R, Zhang Y, Ballas M, et al. PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017;112:200-15. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Lantuejoul S, Damotte D, Hofman V, et al. Programmed death ligand 1 immuno-histochemistry in non-small cell lung carcinoma. J Thorac Dis 2019;11:S89-S101. [Crossref] [PubMed]
- Liu C, Zheng S, Jin R, et al. The superior efficacy of anti-PD-1/PD-L1 immunother-apy in KRAS-mutant non-small cell lung cancer that correlates with an inflamma-tory phenotype and increased immunogenicity. Cancer Lett 2020;470:95-105. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol 2021;18:345-62. [Crossref] [PubMed]
- Sholl LM. Biomarkers of response to checkpoint inhibitors beyond PD-L1 in lung cancer. Mod Pathol 2022;35:66-74. [Crossref] [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-74. [Crossref] [PubMed]
- Carbognin L, Pilotto S, Milella M, et al. Differential Activity of Nivolumab, Pem-brolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One 2015;10:e0130142. [Crossref] [PubMed]
- Daud AI, Wolchok JD, Robert C, et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J Clin Oncol 2016;34:4102-9. [Crossref] [PubMed]
- Fehrenbacher L, von Pawel J, Park K, et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezoli-zumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1156-70. [Crossref] [PubMed]
- Montero MA, Aricak O, Kis L, et al. Clinicopathological significance of the expres-sion of PD-L1 in non-small cell lung cancer. Ann Diagn Pathol 2021;51:151701. [Crossref] [PubMed]
- Vranic S, Gatalica Z. PD-L1 testing by immunohistochemistry in immuno-oncology. Biomol Biomed 2023;23:15-25. [Crossref] [PubMed]
- Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may con-tribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682-8. [Crossref] [PubMed]
- Califano R, Kerr K, Morgan RD, et al. Immune Checkpoint Blockade: A New Era for Non-Small Cell Lung Cancer. Curr Oncol Rep 2016;18:59. [Crossref] [PubMed]
- Song P, Guo L, Li W, et al. Clinicopathologic Correlation With Expression of PD-L1 on Both Tumor Cells and Tumor-infiltrating Immune Cells in Patients With Non-Small Cell Lung Cancer. J Immunother 2019;42:23-8. [Crossref] [PubMed]
- Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with ac-tivating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann On-col 2014;25:1935-40. [Crossref] [PubMed]
- Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 2012;98:751-5. [Crossref] [PubMed]
- Okita R, Maeda A, Shimizu K, et al. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother 2017;66:865-76. [Crossref] [PubMed]
- Takada K, Toyokawa G, Okamoto T, et al. A Comprehensive Analysis of Pro-grammed Cell Death Ligand-1 Expression With the Clone SP142 Antibody in Non-Small-Cell Lung Cancer Patients. Clin Lung Cancer 2017;18:572-582.e1. [Crossref] [PubMed]
- Wu S, Shi X, Sun J, et al. The significance of programmed cell death ligand 1 ex-pression in resected lung adenocarcinoma. Oncotarget 2017;8:16421-9. [Crossref] [PubMed]
- Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocar-cinoma. Onco Targets Ther 2014;7:567-73. [Crossref] [PubMed]
- Cha YJ, Kim HR, Lee CY, et al. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its rela-tionship with p53 status. Lung Cancer 2016;97:73-80. [Crossref] [PubMed]
- Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89:181-8. [Crossref] [PubMed]
- Shah M, Hubbard RA, Mamtani R, et al. Very high PD-L1 expression as a prognostic indicator of overall survival among patients with advanced non-small cell lung can-cer receiving anti-PD-(L)1 monotherapies in routine practice. Pharmacoepidemiol Drug Saf 2022;31:1121-6. [Crossref] [PubMed]
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [Crossref] [PubMed]
- VENTANA PD-L1 (SP263) Assay Staining of Non-Small Cell Lung Cancer Interpretation Guide. Roche Diagnostics 2019:7.
- VENTANA PD-L1 (SP142) Assay Interpretation Guide for Non-Small Cell Lung Cancer ≥50% TC or ≥10% IC Stepwise Scoring Algorithm. Roche Diagnostics 2020:14-5.
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science 2013;339:1546-58. [Crossref] [PubMed]
- Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Car-diovasc Surg 2014;147:921-928.e2. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: interna-tional multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classi-fication of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Miyazawa T, Morikawa K, Otsubo K, et al. Solid histological component of adeno-carcinoma might play an important role in PD-L1 expression of lung adenocarcino-ma. Thorac Cancer 2022;13:24-30. [Crossref] [PubMed]
- Higgins KA, Chino JP, Ready N, et al. Lymphovascular invasion in non-small-cell lung cancer: implications for staging and adjuvant therapy. J Thorac Oncol 2012;7:1141-7. [Crossref] [PubMed]
- Kojima K, Sakamoto T, Kasai T, et al. PD-L1 expression as a predictor of postopera-tive recurrence and the association between the PD-L1 expression and EGFR muta-tions in NSCLC. Sci Rep 2021;11:17522. [Crossref] [PubMed]
- Lin YM, Sung WW, Hsieh MJ, et al. High PD-L1 Expression Correlates with Metas-tasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PLoS One 2015;10:e0142656. [Crossref] [PubMed]
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- Ma Y, Marinkova R, Nenkov M, et al. Tumor-Intrinsic PD-L1 Exerts an Oncogenic Function through the Activation of the Wnt/β-Catenin Pathway in Human Non-Small Cell Lung Cancer. Int J Mol Sci 2022;23:11031. [Crossref] [PubMed]
- Yu W, Hua Y, Qiu H, et al. PD-L1 promotes tumor growth and progression by acti-vating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis 2020;11:506. [Crossref] [PubMed]
- Geng Y, Shao Y, He W, et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem 2015;37:1560-71. [Crossref] [PubMed]
- Zeng DQ, Yu YF, Ou QY, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget 2016;7:13765-81. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018;13:1302-11. [Crossref] [PubMed]


