
A simple and safe surgical technique for nonpalpable lung tumors: One-stop Solution for a nonpalpable lung tumor, Marking, Resection, and Confirmation of the surgical margin in a Hybrid operating room (OS-MRCH)
Highlight box
Key findings
• One-stop solution for a nonpalpable lung tumor, marking, resection, and confirmation of the surgical margin in a hybrid operating room (OS-MRCH) is a novel method for detecting nonpalpable lung tumors and assessing their optimal surgical margins using a simple, one-stop procedure in a hybrid operating room.
What is conventional and what is novel/modified?
• This method of marking nonpalpable lung tumors has the advantage of being safer than using hook wire, faster than bronchoscopic tumor marking, and requires no specialized skills.
• In addition to marking lung tumors, the concurrent confirmation of resection margins allows surgeons to safely complete the surgery.
What is the implication, and what should change now?
• The number of lung resections for nonpalpable lesions is expected to increase and thoracic surgeons will encounter more of them. This method is useful as it can be performed at any facility, is cost-effective, and does not require specialized skills.
Introduction
Ground-glass opacities (GGO) or small lung tumors are generally difficult to diagnose preoperatively and palpate intraoperatively. Especially in video-assisted thoracoscopic surgery (VATS), methods are needed to detect the location of these nonpalpable lung tumors and to ensure sufficient resection margins. The hybrid operating room is a facility where the operating table is seamlessly integrated with a fluoroscope and cone-beam computed tomography (CBCT). It is mainly used for endovascular treatments in the fields of vascular surgery and neurosurgery. We report a simple method that enables both the identification of nonpalpable lung lesions and the confirmation of their resection margins in a single session, without the need for specialized equipment or techniques other thana hybrid operating room. We present this article in accordance with the SUPER reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-25/rc).
Preoperative preparations and requirements
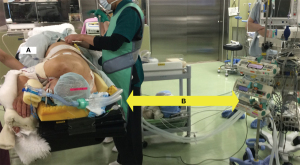
We used a hybrid operating room consisting of an X-ray angiography imaging system and surgical table (Alpheid Hybrid+, INFX-8000H; Canon Medical Systems Corporation, Tochigi, Japan), a stationary digital cardiovascular fluoroscopy system. This system allows CBCT images and angiographies to be obtained on demand during surgery with the patient on the operating table. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Hokkaido University Hospital (No. 022-0102). The requirement for obtaining individual informed consent was waived because of the retrospective nature of the study.
The characteristics of the patients in this study are shown in Table 1. We performed One-stop Solution for a nonpalpable lung tumor, Marking, Resection, and Confirmation of the surgical margin in a Hybrid operating room (OS-MRCH) on 62 unilateral lungs in 60 patients between October 2016 and April 2022. We adopted this method for peripheral lung nodules within approximately 1/3 external of the lung field and for pure/part-solid GGOs or small solid lesions than 1 cm in diameter. Computed tomography (CT) findings showed pure GGOs in 15 cases (24.2%), part-solid GGOs in 22 cases (35.5%), and solid lesions in 25 cases (40.3%). Therefore, lesions presenting part-solid GGO or pure GGO accounted for almost 60% of all lesions. The median size of the whole lesion including GGO was 11.5 mm and that of the solid lesion excluding GGO was 5.3 mm. The median GGO ratio, defined as the solid lesion diameter divided by that of the whole lesion, was 0.51. The median standard uptake value (SUV) on positron emission tomography-CT (PET-CT) was 1.0.
Table 1
| Characteristics | Values (62 lesions in 60 patients) |
|---|---|
| Age (years) | 69 [62–72] |
| Sex | |
| Male | 29 (48.3) |
| Female | 31 (51.7) |
| Side | |
| Right | 36 (58.1) |
| Left | 26 (41.9) |
| Smoking history (pack-years) | 5.5 [0–40.5] |
| Respiratory function (mL) | |
| VC | 3,185 [2,805–3,937] |
| FVC | 3,145 [2,770–3,953] |
| FEV1.0 | 2,335 [1,993–2,763] |
| Comorbidities | |
| Malignant history ≤5 years | 32 (51.6) |
| Diabetes | 15 (24.2) |
| Ischemic heart disease | 4 (6.5) |
| Arrhythmia | 3 (4.8) |
| Autoimmune disease | 3 (4.8) |
| Bronchial asthma | 2 (3.2) |
| Stroke | 1 (1.6) |
| CT findings | |
| Pure GGO | 15 (24.2) |
| Part-solid GGO | 22 (35.5) |
| Solid | 25 (40.3) |
| Tumor size (mm) | 11.5 [8.3–14.8] |
| Solid tumor size (mm) | 6.0 [0.0–8.0] |
| SUVmax on PET-CT | 1.0 [0–1.75] |
Cases of which data are unavailable are excluded. Data are presented as median [IQR] or n (%). VC, vital capacity; FVC, forced vital capacity; FEV1.0, forced expiratory volume in one second; CT, computed tomography; GGO, ground-glass opacity; SUV, standardized uptake value; PET, positron emission tomography.
Step-by-step description
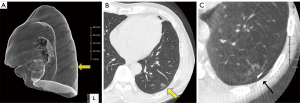
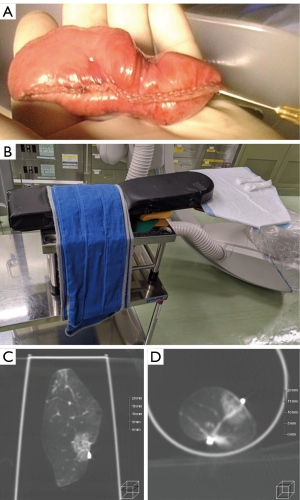
The patient, under general anesthesia and single-lung ventilation, is placed in the decubitus position in a hybrid operating room (Figure 1). After checking for interference between the C-arm of the imaging system and the patient or surgical table, the “Pre-scan” is performed to confirm that the target lesion is captured in the imaging range. The optimal position of the operating table is memorized, and the C-arm is temporarily removed. The patient is then draped and thoracoscopic surgery is initiated as usual. Based on the CT and 3D-CT scan taken prior to the day of surgery (Figure 2A,2B) and considering the location of the intercostal, vertebral, and interlobar fissures, the tumor location is estimated, or based on the results of prior presumptive palpation, the first marking is placed on the lung surface at a site considered to be directly superficial to the lesion by suturing two clips using a LIGAMAX 5 Endoscopic Clip Applier (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) (Video 1). After the thoracoscope is temporarily withdrawn and bilateral ventilation is resumed, a “Marking-scan” is performed to confirm the relationship between the position of the clip-marking and the target lesion (Figure 2C). For successful clip marking, of the GGO, this scan must be performed in the fully inflated as well as Pre-scan. If the location of the clip marking is improper, another clipping and CT scan are needed until the clipping is placed at the location indicating the target lesion correctly. Although most tumors can be located based on either preoperative CT scan or palpation, several Marking-scans may be required if identification is challenging. Alternatively, some cases are more easily identified by palpation than expected by preoperative CT, owing to the natural growth of the tumor. After the placement of the marking clip, single-lung ventilation is resumed, and wedge resection of the lung is performed thoracoscopically using the clip position as a landmark. The resected lung specimen is inflated by injecting air into it using a syringe with a 23- or 25-gauge needle (Figure 3A), after which it is placed in a sealed plastic container. A CT scan of the resected lung, a “Resected-lung scan” is quickly performed in the operating room to confirm the presence of the tumor in the resected specimen and the distance between the resection margin and the tumor is measured (Figure 3B-3D, Video 2). This one-stop procedure enables us to determine whether surgical margins are sufficient on the spot. If the Resected-lung-scan does not ensure a surgical margin of approximately 2–5 mm, it is considered insufficient and additional resection, including partial resection, segmentectomy or lobectomy is performed immediately in the same operating room. If the resection margin is deemed sufficient, the surgery can be completed with reassurance.
Postoperative considerations and tasks
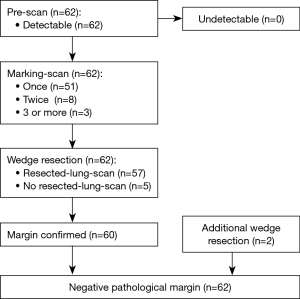
The results for the 62 cases that underwent OS-MRCH are shown in Table 2. The median operative time was 85.0 minutes (range, 41–245 minutes), and of the 62 cases, 19 (30.6%) underwent uniportal VATS, 43 (69.4%) underwent multi-portal VATS, and no cases underwent thoracotomy. The postoperative diagnoses were primary lung cancer in 45 cases (72.6%), metastatic lung tumor in 15 (24.2%), and benign tumor in two (3.2%). In all 62 cases, the location of the lung lesion was successfully confirmed in the Pre-scan, indicating that CBCT was satisfactorily accurate, and its images had adequate resolution. The average number of Marking-scan taken between the initial clipping and resection was 1.8, with 51 cases (82.3%) requiring one, eight cases requiring two, and three cases where the tumor locations were challenging to identify needing three or more scans. The maximum number of Marking-scan was four. Among the 62 cases who underwent partial lung resection, Resected-lung-scan was performed in 57 (91.9%). In five cases in which the resection margin was clearly identified via palpation, the Resected-lung-scan step was skipped. In 60 cases (96.8%), the resection margin was judged to be sufficient on the Resected-lung-scan. In two cases the margin was determined to be insufficient and additional partial lung resection was immediately performed. Negative resection margins were confirmed pathologically in all 62 cases (Figure 4). One case with mucinous was diagnosed pathologically and underwent a secondary completion lobectomy at a later date. The final procedure in 44 of the 45 primary lung cancer cases was wedge resection not lobectomy, which included both “radical reduced surgery”, for small peripheral lung cancer, and “palliative reduced surgery” for the patients intolerant of lobectomy due to such factors as poor respiratory function. Regarding postoperative complications, prolonged air leak was observed in two cases, and chest wall bleeding in one case. No unique complications were attributed to this method, including air embolization owing to clipping (Table 2).
Table 2
| Variables | Values (n=62) |
|---|---|
| Operating time (minutes) | 85.0 [69.3–116.5] |
| Blood loss (mL) | 0 [0–0] |
| No. of the wound | |
| One | 19 (30.6) |
| Two | 22 (35.5) |
| Three | 21 (33.9) |
| No. of Marking-scan for tumor detection | |
| Once | 51 (82.3) |
| Twice | 8 (12.9) |
| 3 or more | 3 (4.8) |
| Tumor detection | |
| Yes | 62 (100.0) |
| No | 0 |
| Resected-lung scan | |
| Yes | 57 (91.9) |
| No | 5 (8.1) |
| Additional wedge resection | |
| Yes | 2 (3.2) |
| No | 60 (96.8) |
| Complications | |
| Yes | 3 (4.8) |
| Prolonged air leak | 2 |
| Chest wall bleeding | 1 |
| No | 59 (95.2) |
| Pathology | |
| Primary lung cancer | 45 (72.6) |
| ADC | 40 |
| Sq | 4 |
| SCLC | 1 |
| Metastatic lung tumor | 15 (24.2) |
| Benign tumor | 2 (3.2) |
| Pathological tumor size (mm) | |
| Whole size | 12.0 [8.0–15.0] |
| Invasive size | 7.0 [0–9.0] |
| Pathological margin | 11.0 [7.0–15.3] |
Data are presented as median [IQR] or n (%). OS-MRCH, one-stop solution for a nonpalpable lung tumor, marking, resection, and confirmation of the surgical margin in a hybrid operating room; ADC, adenocarcinoma; Sq, squamous cell carcinoma; SCLC, small cell lung carcinoma.
Tips and pearls
To ensure the detection of GGO lung tumors using CBCT, the anesthesiologist should sufficiently inflate the lungs during the Pre-scan and Marking-scan procedures. Because the Marking-scan is performed after single-lung ventilation, it is important to note whether the lungs are sufficiently inflated. Second, when injecting air into the specimen prior to the Resected-lung-scan, it is crucial to use the smallest-gauge needle possible and not inject too rapidly, to prevent emphysematous damage in the lung parenchyma, which can make it difficult to assess the margin precisely. Imaging should be promptly performed following air injection to prevent deflation of the Resected lung. Third, during the clip-marking procedure, a suture needle should be inserted only on the surface of the lung because a deep insertion may lead to hematoma and make it difficult to recognize the GGO in the Marking-scan. Finally, the clip should be attached to the ends of the suturing thread before stitching and secured using another clip. This procedure does not require ligation and reduces suturing time.
Discussion
With recent advances in imaging technology, nonpalpable lung tumors such as GGO, peripheral small lung cancers, and metastatic lung tumors, are being identified more frequently and opportunities for the surgical resection of these lesions are increasing. Furthermore, considering the results of studies such as the CALGB140504 trial, there is a growing demand for techniques and methods to reliably perform sublobar resections, especially for peripheral small lung tumors (1). Since thoracoscopic surgery has become the standard procedure, preoperative or intraoperative marking is required for the resection of such lung lesions, and various methods have been developed and reported.
Percutaneous CT-guided marking with hook wire has been used since the 1990s (2-4). Although this method has the advantage of being performed preoperatively using high-resolution CT, not a few cases of fatal air embolisms have been reported, and some safety issues have been noted with it (4-6). Percutaneous CT-guided marking using microcoil or lipiodol is a safer method, with a lower incidence of complications, such as pneumothorax and air embolism than using hook wire (7). To ensure safe and accurate localization, several methods have been developed using a bronchoscopic approach to place dyes or markers near the tumor through the airways, such as virtual assisted lung mapping (VAL-MAP) (8-11) or radiofrequency identification (RFID) marking system (12-15). These methods ensure the precise identification of nonpalpable lung tumors and safe margins for resection, and their feasibility and applicability in lung segmentectomy have been reported. Although these preoperative bronchoscopic marking methods are safe and highly effective, they require proficiency in bronchoscopic techniques (11) and RFID-specific equipment (14). Furthermore, there is a potential risk of the RFID tag being dislocated (14).
The major advantage of our proposed method is its simplicity. It can be performed in the operating room as a one-stop procedure, from tumor marking to confirmation of the resection margin, and it requires no specialized skills or equipment as long as a hybrid operating room is available. This simplicity makes it possible for almost any thoracic surgeon to perform the procedure. In addition, preoperative bronchoscopy may impose mental and physical stress on patients, but our method does not possess this shortcoming.
Another problem regarding surgery for nonpalpable lung tumors is securing the resection margin, as inadequate surgical margins are inevitable risk factors for local recurrence. VAL-MAP 2.0 identifies appropriate deep lung resection margins by implanting microcoil (16). In the RFID method, the tag is implanted at the same position as the tumor, allowing the margin to be estimated by scanning the resected lung (15). However, there are few reports on using of CT scans to confirm the resection margins of GGO lung tumors. Mazza et al. reported a method of superficial and deep edge marking in a hybrid operating room during surgery for nonpalpable lung tumors (17), and our method is a more simplified version of their method eliminating the need for the bronchoscopic marking by CBCT scanning of resected lung to confirm the deep margin. High-resolution CT scans performed in the examination room are considered more useful than CBCTs for confirming the resection margins of GGO. However, the timing of the examination and the time until imaging is possible following lung inflation can be problematic. By comparison, our method has the advantage that resection margins can be confirmed immediately following resection within the same operating room.
Another concern is that marking methods placing an object such as a microcoil near the tumor can have potentially negative impacts on the postoperative pathological diagnosis. By contrast, our method has almost no effect on the pathological diagnosis because the margin can be confirmed without damaging the resected specimen.
One of the disadvantages of our method is the additional radiation exposure to the patient and medical staff. At least three CBCT scans, including a Pre-scan are required, additionally, in cases of difficult-to-identify lesions, four or more CT scans may be required before resection. Fibrosis due to interstitial pneumonia or emphysematous changes due to smoking makes it more challenging to identify the location of the lung nodule, and preoperative smoking cessation might reduce tumor size (18). Even for the Resected-lung-scan, externally performed, patient exposure to radiation is unavoidable. Although CBCT is known to deliver lower radiation doses than helical CT, accurate evaluation of the dose is difficult because it is affected by the physical size and area of the body being measured. The radiation dose was 0.57× lower than that of helical CT in a study conducted by Mitsuoka et al. using a phantom (19); however, radiation exposure should be reduced as much as possible in order to minimize the health risks to patients and medical staff. The preoperative bronchoscopic markings described above are usually performed under fluoroscopic guidance, and a confirmatory helical CT or CBCT is sometimes performed after these markings. Consequently, radiation exposure cannot be avoided using these methods either (20). Considering these factors, the overall radiation exposure from our method is not expected to be significantly higher than what is used in bronchoscopic marking methods.
Another limitation of our method is the restrictions caused by the operating table system and C-arm. The table in the hybrid operating room is generally narrower than that in a standard operating room, and the C-arm of the CBCT must maintain a certain rotation range. Therefore, patients in the decubitus position must have their arms bent to keep their bodies positioned within the rotational range of the C-arm. In addition, various lines connecting the patient and anesthesia area, such as a corrugated tube, monitoring cords and intra-venous/arterial lines, must be bundled along the table to avoid interference with the rotation of the C-arm. It is challenging to capture the target lesion within the imaging range of CBCT, particularly in large patients or when the tumor is located extremely ventrally or dorsally. These disadvantages result in relatively long operating time. The need to connect the patient to the ventilator with a corrugated tube that is more than twice the standard length unavoidably increases the apparatus dead space in the ventilator circuit. Therefore, using this method in pediatric patients is severely affected by the significant dead space and is considered high-risk. Another concern with CBCT is that its resolution is potentially insufficient to detect GGO tumors (21); however, none of the lesions in our cases proved undetectable, including pure GGO lesions. The results are consistent with the report by Mitsuoka et al. (22) that 145 lesions, including pure GGO, were identified 100% by CBCT, indicating that CBCT is sufficient to detect GGO and small lung nodules.
We have already advanced this method to further stages by capitalizing on its advantages and applying it to partial lung resections other than GGOs or small lung tumors. During lung resection in patients with interstitial pneumonia, for example, it is sometimes difficult to palpate not only GGO tumors but also solid nodules because the entire background lung is obscured by fibrosis. We have applied our method to confirm the extent of resection in advance for such cases, by performing a Marking-scan after placing clips at 2–3 points over the entire resection area, rather than directly above the tumor. In partial lung resection of lung tumors with predominantly cavitary lesion, palpation can also be difficult even when the tumor is located close to the pleural lung surface. Our novel method is also useful in these cases, as surgery can be performed free from the stress of being unable to identify the lesion.
The opportunities for resecting nonpalpable lung tumors will continue to increase. Therefore, the prevalence of hybrid operating rooms and standardization of techniques aimed at shortening operative time are necessary to ensure the stability of this method.
Conclusions
This novel lung tumor marking method “OS-MRCH”, is simple and useful for detecting nonpalpable lung tumors. It has the potential to become an effective marking technique for lung tumors, with high rates of tumor detection, successful marking, and complete pathological resection. With the increasing need for partial resections of pulmonary nodules, this method is expected to become increasingly valuable.
Acknowledgments
The authors would like to thank Editage (https://www.editage.jp/) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-25/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-25/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-25/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Hokkaido University Hospital (No. 022-0102) and performed in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for obtaining individual informed consent was waived because of the retrospective nature of the study; however, information about this study was made readily available to the legal guardians of the patients on the institutional website.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Kanazawa S, Ando A, Yasui K, et al. Localization of pulmonary nodules for thoracoscopic resection: experience with a system using a short hookwire and suture. AJR Am J Roentgenol 1998;170:332-4. [Crossref] [PubMed]
- Yoshida Y, Inoh S, Murakawa T, et al. Preoperative localization of small peripheral pulmonary nodules by percutaneous marking under computed tomography guidance. Interact Cardiovasc Thorac Surg 2011;13:25-8. [Crossref] [PubMed]
- Yi JH, Choi PJ, Bang JH, et al. Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review. J Thorac Dis 2018;10:E59-64. [Crossref] [PubMed]
- Oikawa T, Nomoto Y, Kinoshita K. A case of left-sided hemiplegia due to cerebral air embolism caused by CT-guided marking for small peripheral lung cancer. The Journal of the Japanese Association for Chest Surgery 2008;22:914-9.
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Sato M, Murayama T, Nakajima J. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP). J Thorac Dis 2016;8:S716-30. [Crossref] [PubMed]
- Sato M, Kuwata T, Yamanashi K, et al. Safety and reproducibility of virtual-assisted lung mapping: a multicentre study in Japan. Eur J Cardiothorac Surg 2017;51:861-8. [Crossref] [PubMed]
- Tokuno J, Chen-Yoshikawa TF, Nakajima D, et al. Improved visualization of virtual-assisted lung mapping by indocyanine green. JTCVS Tech 2021;10:542-9. [Crossref] [PubMed]
- Yutaka Y, Sato T, Zhang J, et al. Localizing small lung lesions in video-assisted thoracoscopic surgery via radiofrequency identification marking. Surg Endosc 2017;31:3353-62. [Crossref] [PubMed]
- Sato T, Yutaka Y, Nakamura T, et al. First clinical application of radiofrequency identification (RFID) marking system-Precise localization of a small lung nodule. JTCVS Tech 2020;4:301-4. [Crossref] [PubMed]
- Yutaka Y, Sato T, Tanaka S, et al. Feasibility study of a novel wireless localization technique using radiofrequency identification markers for small and deeply located lung lesions. JTCVS Tech 2022;12:185-95. [Crossref] [PubMed]
- Yutaka Y, Ohsumi A, Nakajima D, et al. Intraoperative margin assessment by wireless signals in thoracoscopic anterior (S3) segmentectomy using a radiofrequency identification marker. Gen Thorac Cardiovasc Surg 2022;70:509-13. [Crossref] [PubMed]
- Sato M, Nagayama K, Kobayashi M, et al. Virtual-Assisted Lung Mapping 2.0: Preoperative Bronchoscopic Three-Dimensional Lung Mapping. Ann Thorac Surg 2019;108:269-73. [Crossref] [PubMed]
- Mazza F, Venturino M, Peano E, et al. Single-Stage Localization and Thoracoscopic Removal of Nonpalpable Pulmonary Nodules in a Hybrid Operating Room. Innovations (Phila) 2020;15:555-62. [Crossref] [PubMed]
- Maci E, Comito F, Frezza AM, et al. Lung nodule and functional changes in smokers after smoking cessation short-term treatment. Cancer Invest 2014;32:388-93. [Crossref] [PubMed]
- Mitsuoka M, Terazaki Y, Okamoto Y, et al. Intraoperative marking of an impalpable pulmonary nodule using the Hybrid OR System. The Journal of The Japanese Association for Chest Surgery 2015;29:552-8.
- Verhoeven RLJ, van der Sterren W, Kong W, et al. Cone-beam CT and Augmented Fluoroscopy-guided Navigation Bronchoscopy: Radiation Exposure and Diagnostic Accuracy Learning Curves. J Bronchology Interv Pulmonol 2021;28:262-71. [Crossref] [PubMed]
- Ohtaka K, Takahashi Y, Kaga K, et al. Video-assisted thoracic surgery using mobile computed tomography: new method for locating of small lung nodules. J Cardiothorac Surg 2014;9:110. [Crossref] [PubMed]
- Mitsuoka M, Kashihara M, Nishi T, et al. Cone-Beam Computed Tomography-Guided Marking of Small Pulmonary Nodules with Surgical Clips. Kurume Med J 2023;68:183-9. [Crossref] [PubMed]


