
Biological characteristics and clinical treatment of pulmonary sarcomatoid carcinoma: a narrative review
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare type of non-small cell lung cancer (NSCLC) with a poor prognosis even when diagnosed at early stages (1) and usually occurs in older males (2). According to the 2021 World Health Organization (WHO) classification, PSC can be classified as pulmonary pleomorphic carcinoma (PPC), spindle cell carcinoma (SCC), giant cell carcinoma (GCC), carcinosarcoma, and pulmonary blastoma (PB) (3). The complex composition of PSC arises from the coexistence of epithelial and sarcomatoid components, varying in proportions and types within the tumor tissue. The origin of these complex components is essential for understanding PSC and developing therapeutic strategies against it. Nevertheless, the rarity of PSC and its intra- and inter-tumor heterogeneity present substantial challenges in clarifying its biological characteristics.
The treatment for advanced PSC has followed the standard first-line approach for NSCLC, yet the outcomes have proven unsatisfactory, and PSC exhibits high resistance to chemotherapy and radiotherapy (4). For patients with NSCLC, directed targeted therapies based on aberrant gene mutation have significantly improved survival (5). There is a significant amount of evidence indicating that PSC has a high mutation frequency (6,7), and thus targeted therapy for mutation-positive patients may have considerable potential. Additionally, immune checkpoint inhibitors (ICIs) have yielded promising results in NSCLC in recent years, and PSC exhibits a high level of immune infiltration and expression of immune checkpoints (8). Overall, immunotherapy, particularly the administration of ICIs, represents a new hope for patients with PSC.
In this paper, we discuss the histologic transformation, genetic characteristics, metabolism, and immune microenvironment of PSC, and review various therapeutic strategies, including chemotherapy, targeted therapy, and immunotherapy. We hope to provide insight and promote a need for a deeper understanding of this rare and highly malignant lung tumor. We present this article in accordance with the Narrative Review reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-127/rc).
Methods
We have systematically searched the PubMed database using the following search terms: “pulmonary sarcomatoid carcinoma”, “genetic mutations”, “immune microenvironment”, “hypoxia”, “angiogenesis”, “overall survival”, “surgery”, “radiotherapy”, “chemotherapy”, and “immune checkpoint inhibitors”. Only original articles published in English, including research articles and literature reviews, were included in this review (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | August 29, 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | “pulmonary sarcomatoid carcinoma”, “genetic mutations”, “immune microenvironment”, “hypoxia”, “angiogenesis”, “overall survival”, “surgery”, “radiotherapy”, “chemotherapy”, “immune checkpoint inhibitors” |
| Timeframe | Studies published from July 16, 1990 to August 29, 2023 |
| Inclusion and exclusion criteria | Inclusion criteria: English language and original publications, including research articles and literature reviews |
| Exclusion criteria: non-English language and no full-text available | |
| Selection process | Study selection and assessment were conducted by the first author Y.W. and the other authors consented to the selection process |
Biology of PSC
Origin and histological transformation
PSC has gained much attention due to the coexistence of its epithelial and sarcomatoid components. As early as 1863, Virchow initially described carcinosarcoma and posited a theory concerning the origin of its two components: they either occur separately and later intermix, or one component transforms into another during tumor development (9). Over time, it became the prevailing belief that PSC originated from carcinoma, with the epithelial components dedifferentiating into the sarcomatoid components. The transition areas from carcinoma to sarcomatoid component were revealed by light and electron microscopy (10).
There have been reports in some individual patients of transformation from common NSCLC to PSC (11-13) or even from SCLC to PSC (14,15). The morphology and immunohistochemistry of PSC also suggest an NSCLC origin, typically the lung adenocarcinoma (LUAD) and lung squamous carcinoma (LUSC) subtypes (16). Vieira et al. explored the possible origin of PSC by immunohistochemical analysis of thyroid transcription factor 1 (TTF1) expression in LUAD and P63 expression in LUSC, reporting that 41.5% and 17% of the PSC cases originated from LUAD and LUSC, respectively (17). Through whole-exome sequencing (WES), Yang et al. analyzed the somatic mutations and copy number variations (CNVs) in 56 patients with PSC and found clonal correlations between two components in all patients by calculating the tumor clonality index (CI) (8). These results suggested a common origin of the epithelial and sarcomatoid components of PSC (8).
Epithelial-mesenchymal transition (EMT) plays a crucial role in tissue fibrosis, cancer development, progression, and metastasis (18-20). Cells lose cell adhesion and polarity, acquiring mesenchymal features that are similar to the morphology and migratory capacity of spindle cells (21). The coexistence of the more differentiated epithelial component and the less differentiated sarcomatoid component potentially suggests that PSC originates from the transition of malignant epithelial cells into a mesenchymal phenotype (22-24). Several studies have shown that PSC undergoes an EMT process (25-27). In one study, transcriptomic data from the 195 differentially expressed genes (DEGs) of both components primarily displayed enrichment in epithelial, mesenchymal features, and EMT-related pathways (8). Manzotti et al. analyzed 146 differential genes using Gene Ontology enrichment analysis, which identified sarcomatoid components enriched for downregulated genes mainly related to cell adhesion and epithelial differentiation during the epithelial-to-sarcomatoid transition (28); and enriched for upregulated genes involved in extracellular interactions and angiogenesis (28). DNA methylation data also supports a connection between PSC and EMT, indicating the potential involvement of DNA methylation in EMT regulation (8).
Immunohistochemical research suggests that the expression of EMT-related markers such as E-cadherin, vimentin, Twist-related protein 1 (Twist1), Zinc finger E-box-binding homeobox 1 protein (ZEB1), Slug, Snail, c-Jun, and vasculogenic mimicry (VM) are increased in PSC compared to LUSC (29-32). ZEB1 is sensitive and highly specific for PSC diagnosis (33), predicting a poor prognosis (31). There is no difference in immunohistochemical vimentin expression between PSC biopsy and surgical specimens, indicating the prevalence of the EMT process in PSC (34). In addition, the PSC EMT process may be connected to integrin-related pathways, with Shimizu et al. suggesting that overexpression of fibronectin mediated by the integrin ligase kinase (ILK) signaling pathway contributes to the EMT transformation of PSC (35).
Overall, most evidence supports a common origin of PSC’s complex histologic composition, with EMT figuring prominently in this process. However, further research is still needed to confirm these conclusions.
Genetic characteristics
PSC exhibits a high mutation level (6,7) compared to other NSCLC subtypes (36,37) (Figure 1). In their study, Schrock et al. reported that the mean tumor mutational burden (TMB) of PSC of 13.6 mutations per megabase (Mb), with 20% of cases having a TMB of >20 mutations/Mb and 43% having a TMB of >10 mutations/Mb (38). The WES results from Liu et al.’s analysis of 10 cases included a total of 1461 somatic mutations, of which 58.2% were missense mutations, 27.0% silent mutations, 5.3% deletion mutations, 5.2% nonsense mutations, 2.3% insertion mutation, 1.8% splice-site mutations, and 0.3% stop-loss mutations (39). Patients with a smoking history are more likely to carry mutations (17), and the high mutation rate of PSC may be related to genetic instability due to tobacco exposure (40).
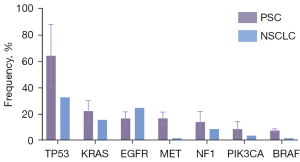
The most frequent mutations in PSC are tumor protein 53 (TP53), Kirsten rat sarcoma viral oncogene homolog (KRAS), epidermal growth factor receptor (EGFR), and mesenchymal to epithelial transition factor (MET) (7,8,17,36-38,41-44). Yang et al. performed comprehensive WES of 56 patients with PSC (8). It was found that TP53 was the most common mutation (44/56), 57% of patients carried mutations in the RTK/RAS pathway gene, 16% in EGFR, 14% in KRAS, 13% in MET, 7% in BRAF, 5% in NF1, and 4% in NRAS. Most EGFR mutations, such as exon 19 deletion and exon 21 L858R, were common and targetable. KRAS mutations mainly involved codon 12. MET mutations were mostly MET exon 14 skipping alterations. Mutations in the phosphatidylinositol 3 kinase (PI3K) pathway were present in 27% of patients: 13% were PIK3CA, 9% PTEN, 5% AKT3, and 2% AKT1. Schrock et al. detected 125 mutated genes in formalin-fixed paraffin-embedded (FFPE) PSC samples, including TP53 (74%), KRAS (34%), MET (13.6%), EGFR (8.8%), BRAF (7.2%), HER2 (1.6%), and RET (0.8%) (38). Fallet et al. tested 114 surgical samples, revealing that the most common mutations were KRAS (27.2%), EGFR (22.2%), TP53 (22.2%), STK11 (7.4%), NOTCH1 (4.9%), NRAS (4.9%), and PI3KCA (4.9%) (7). The variability in mutation sites and frequencies detected across different panels reflects differences in sample size and the sensitivity of detection methods.
Biphasic PSC represents the most prevalent subtype of PSC. To investigate the homogeneity and heterogeneity of gene mutations between the two components of biphasic PSC, Liu et al. performed laser capture microdissection and next-generation sequencing (NGS) on 31 PSC samples (45). The most frequently altered genes in epithelial components were TP53 (74%), MET (19%), EGFR (19%), KRAS (19%), NF1 (19%), and MAP3K1 (19%); meanwhile, the most frequently altered genes in sarcomatoid components were TP53 (74%), MET (23%), EGFR (19%), KRAS (19%), and NF1 (19%). Notably, 97% of patients exhibited common mutations and frequencies in both components. Similarly, WES analysis of 15 cases by Pécuchet et al. demonstrated that the most common driver mutations in PSC are predominantly in the main clonal population, with subclonal populations accounting for less than 10% (46), implying a monoclonal origin of PSC.
Metabolic microenvironment
It is well known that a hypoxic tumor microenvironment (TME) is an inherent feature of solid malignant tumors and is widely recognized as an independent indicator of poor prognosis (47,48). Hypoxia affects various aspects of tumor cell metabolism and angiogenesis, promoting tumor heterogeneity and plasticity (47,49,50). Compared with other NSCLC subtypes, PSC has a higher level of hypoxia (51).
Hypoxia-inducible factor (HIF), a heterodimer with multiple isoforms (52), predominantly includes HIF-1α, which is responsible for activating transcriptional responses under hypoxic conditions and promoting tumor progression (53). Chang et al. assessed the expression of HIF-1α in 122 cases of PPC, with 92 (75.4%) displaying the overexpression of HIF-1α (54). The expression was concentrated in the nucleus of the perinecrotic region of the sarcoma component (54). In an analysis of 55 cases each of PPC and LUAD, Tsubata et al. found that the HIF-1α expression levels were significantly higher in PPC than in LUAD (P=0.03) (51). Multivariate analysis in another study suggested that extensive tumor necrosis is an independent prognostic factor for PSC (55). These findings are consistent with the clinical observation that PSC tends to involve a larger tumor size and is associated with more necrotic components.
Under a hypoxic environment, tumor cells adapt by autonomously altering various metabolic pathways, such as that seen in the Warburg effect, to meet their increased bioenergetic and biosynthetic needs (56). HIF-1α performs an important role in redirecting glucose metabolism patterns from oxidative phosphorylation to glycolysis in tumor cells (57).
PSC is considered to be a type of cancer with high metabolic levels. 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT), a valuable diagnostic tool for malignant tumors based on glucose metabolism, has confirmed the high glucose metabolism levels in the PSC (58). The reported standardized uptake values (SUVs) for the positron emission tomography of 18F-FDG in patients with PSC tend to be high (median 19.3) (59), which is significantly higher than those of other NSCLC subtypes (60,61).
Enhanced glucose uptake in tumor cells is facilitated by glucose transporter carriers (62), which have significantly increased in tumor tissues (63,64). HIF-1α can induce the expression of glucose transport proteins such as glucose transporter 1 (Glut1) and Glut3 (57). The overexpression of Glut1 in human tumors is closely related to 18F-FDG uptake (65,66). Research indicates higher Glut1 expression in undifferentiated cancers compared to well-differentiated cancers (67), as in PSC. In one study, immunohistochemical staining of 104 patients with PSC revealed that 48% of samples had a high Glut1 expression, which was significantly associated with vascular infiltration and tumor cell proliferation (68). Additionally, the univariate analysis indicated that Glut1 was a significant predictor of poorer postoperative overall survival (OS), with those patients showing high Glut1 expression experiencing a worse prognosis than those with low expression (68).
Angiogenesis and vascular invasion are critical biological processes in tumor development and metastasis (69-73). The balance between proangiogenic and antiangiogenic factors is disrupted during tumor development due to hypoxia and an insufficiency in metabolic substances (74). HIF induces the expression of several proangiogenic factors, such as vascular endothelial growth factor (VEGF) and VEGF receptor (75).
Microvessel density (MVD) can be used to assess tumor angiogenesis, which correlates with the expression of angiogenic factors, such as VEGF, and is predictive of a poor prognosis in NSCLC (76,77). In an analysis of 55 cases each of PPC and LUAD, Tsubata et al. found that MVD expression levels were significantly higher in PPC than in LUAD (P=0.03) (51). Hypoxia-induced overexpression of HIF-1α activates VEGF transcription, leading to a poorer prognosis and increased chemoresistance (78,79), which is a potential cause of the chemoresistance in PSC. The incidence of vascular invasion in NSCLC is usually 30–50% (55,80,81) but is 90% in patients with PSC (82). Vascular invasion can be an independent factor for poor prognosis (17,83).
PSC is a hypoxic and hypermetabolic type of tumor, with frequent angiogenesis and vascular invasion. These characteristics are also relevant to therapy resistance (54). Hence, comprehensively characterizing the metabolic features of PSC, along with its two components, is critical to devising effective treatments.
Immune microenvironment
The composition of the tumor immune microenvironment (TIME) is integral to governing tumor-immune interactions. Most studies point to PSC being a “hot tumor”. Moreover, a higher TMB and leukocyte fraction indicate a T cell-inflamed microenvironment in the PSC (8).
Programmed cell death protein 1/programmed cell death-ligand 1 (PD-1/PD-L1), the most important immune checkpoint, has a high expression in PSC (84,85). In one study, the immunohistochemical results for PD-L1 were positive in 95% of patients, with a median PD-L1 expression of 70% (37). Kim et al. assessed PD-L1 expression in patients included in a clinical trial (NCT03022500), with 64.3% of the patients exhibiting PD-L1 expression ≥50% (41). PD-L1 expression is higher in the sarcomatoid component compared to the epithelial component (86-88). In the multivariate analysis of one study, high PD-L1 expression correlated with a better prognosis (89). Zhou et al.’s study revealed that the PD-L1 tumor proportion scores (TPSs) of <1%, 1–49%, and ≥50% corresponded to overall response rates (ORRs) of 33.3%, 72.7%, and 85.7%, and median progression-free survival (PFS) of 6.0, 6.7, and 10.3 months, respectively (90). In a different study, a higher TMB compared to a lower TMB was correlated with higher PD-L1 expression and median OS (18 vs. 1.84 months) (37).
Lymphoid and myeloid cells constitute the majority of immune cells in the TME. Vieira et al. reported that the expression level of CD3 was higher in PSC than in NSCLC, suggesting greater T-cell infiltration in PSC (91). Immunohistochemistry in another study confirmed the enrichment of CD8+ T cells in the PSC (92). Meanwhile, Zhang et al. reported that PD-L1 expression was positively correlated with CD8+ T-cell infiltration in patients with PSC (93). Tumor infiltration CD8+ T is more prevalent in PD-L1-positive patients with PSC than in PD-L1-negative patients (87). Chen et al.’s analysis of FFPE samples from 100 patients found that those with higher levels of CD8+ T-cell infiltration experienced better prognosis than did those with lower levels of infiltration (mean OS 92.3 vs. 31.2 months; P<0.05) (94). In analyses of the baseline circulating lymphocyte composition of PSC, patients who experienced treatment benefits had a higher proportion of CD8+ T cells compared to those who did not receive benefits (41).
Myeloid cells also extensively infiltrate PSC. Immunohistochemistry suggests that tumor-associated macrophages (TAMs) are enriched in PSC (91,95), which is potentially related to the EMT process. In one study, CD163+ macrophages (M2 type) showed greater infiltration in PSC than in NSCLC and correlated with high PD-L1 expression (91). Furthermore, an elevated neutrophil-to-lymphocyte ratio (NLR) in peripheral blood correlates with poorer PSC prognosis (96). As for the difference in immune infiltration of two components of PSC, Yang et al. conducted a fluorescent multiplex immunohistochemical analysis of PD-L1, CD4, CD8, CD68, and FoxP3 between the two components of PSC and found no significant difference between them (8).
Some studies have performed immunophenotyping to investigate the immune microenvironmental characteristics of PSC. Ma et al. classified 32 patients with PSC into three types based on their infiltration with CD8+ T cells in different spatial regions (intratumoral and peritumoral regions), classifying 65.6%, 15.6%, and 18.8% as immune-inflamed, immune-excluded, and immune-desert, respectively. (92) Combining intratumoral CD8+ T-cell infiltration and the TPS of PD-L1 staining in the intratumoral region, they then classified patients into four types: type I (PD-L1+/CD8+: adaptive immune resistance), type II (PD-L1−/CD8−: immunologic ignorance), type III (PD-L1+/CD8−: intrinsic induction), and type IV (PD-L1−/CD8+: tolerance). The type I immunophenotype was the most common subtype in PSC, accounting for 46.9% of patients (92). Yang et al. generated three clusters of patients with PSC based on DNA methylation data, with the cluster that was most enriched in patients having the lowest level of DNA methylation and the greatest degree lymphocyte infiltration (8).
As PSC is a hot tumor, with rich infiltration of immune cells and high expression of immune checkpoints, it likely has an adaptive immunosuppressive microenvironment. Therefore, the prospects for immunotherapy, particularly ICIs, are promising for patients with PSC.
Clinical characteristics and treatment
Clinical characteristics
PSC tends to occur in older males with a smoking history (4,37,93,97). Patients are often diagnosed at an advanced tumor stage (98) and are accompanied by clinically significant symptoms (2), including cough, sputum, hemoptysis, chest pain, and weight loss. PSC usually has a large tumor size (99), with marked central necrosis (100). Imaging diagnostics frequently reveal a central low attenuation area or cavity (101). Biphasic tumors (4,102), particularly PPC, are more prevalent in PSC (2,7,103). Metastases occur early and frequently in patients with PSC, with the common sites being similar to those of NSCLC (adrenal glands, lungs, pleura, brain, bone, or liver). Over half of the patients with PSC exhibit metastases in more than two locations (4,100). The median OS for patients with PSC is approximately 6.3 months, while the median PFS is 2 months (37). The 1-, 2-, and 5-year survival rates are approximately 42%, 23%, and 15%, respectively (98).
Surgical treatment
Patients with early-stage lung cancer, including PSC, can benefit from surgical treatment. The survival and prognosis of patients with PSC who undergo surgical treatment are significantly better than those who do not (59,104). In a retrospective analysis of 400 patients with PSC from the Surveillance, Epidemiology, and End Results (SEER) database, Xie et al. found that patients who received surgical treatment exhibited a more favorable prognosis compared to those who did not [hazard ratio (HR) =1.43] (105). Moreover, the median survival time was better in postoperative patients (23.0 vs. 11.0 months; P=0.016) (104). It is worth noting that the 5-year survival rate for postoperative patients with PSC ranges from 11% to 24.5% (106), which is lower than that of patients with other types of NSCLC, suggesting the highly malignant and aggressive nature of PSC.
For patients with early-stage PSC, surgical treatment is the preferred treatment approach. However, a significant proportion of patients with PSC are not suitable for surgery, making medical therapy a necessary option. PSC is associated with a high postoperative recurrence risk and thus has a shorter survival time compared with other types of NSCLC. Consequently, it is critically important to consider adjuvant therapy following surgery.
Chemotherapy
For resectable NSCLC, surgery remains the most essential treatment strategy, while chemotherapy or radiotherapy serves as adjuvant therapy. For advanced NSCLC, chemotherapy nonetheless retains an indispensable role. However, several cases suggest that PSC is insensitive to chemotherapy (58,107-109).
First-line chemotherapy
In a retrospective study with 71 advanced patients with PSC receiving first-line chemotherapy, 73% received platinum-based chemotherapy (4). The outcomes showed that 16.5% of patients achieved a partial response (PR), 14.5% had stable disease (SD), and 69% experienced disease progression (PD) (4). Among all patients, the median PFS was 2.0 months, and the median OS was 6.3 months (4), which was lower compared to that of other NSCLC subtypes (In three randomized studies of 984 patients with non-PSC NSCLCs, the median PFS was 4.3 months, and the median OS was 8.9 months) (110). Another retrospective study included 28 patients with advanced or recurrent PSC, 85% of whom received platinum-based therapy (2). In this cohort, the ORR was 7%, 21% had SD and 72% exhibited PD (2). The median time to progression (TTP) was 2.7 months, and the median OS was 4.3 months (2). A phase II study investigating carboplatin plus paclitaxel with or without bevacizumab enrolled 16 patients with PSC. Among them, seven patients received carboplatin plus paclitaxel alone, resulting in a 0% ORR (SD: 2; PD: 4; not evaluable: 1), a median PFS of 1.2 months, and a median OS of 7.9 months (111). In another study, patients treated with platinum-based chemotherapy had a better OS than those treated with non–platinum-based chemotherapy (7.0 vs. 5.3 months; P=0.096) (4), which was corroborated by another study (5.6 vs. 1 month) (2). The multivariate analysis of the former study indicated that platinum-based chemotherapy (HR =0.92) was associated with better OS than non–platinum-based chemotherapy (4).
Targeting the sarcomatoid component may be a viable strategy for PSC treatment. The regimen of combined chemotherapy, including mesna, doxorubicin, ifosfamide, and dacarbazine (MAID), has demonstrated efficacy in soft tissue sarcoma (112,113). There are reports of positive responses in patients with PSC treated with this regimen (114). Lee et al. retrospectively analyzed 17 patients with PSC treated with MAID and reported an ORR of 35%, a median PFS of 2.8 months, and a median OS of 8.7 months (115). Therefore, developing therapeutic strategies that specifically target the sarcomatoid component has potential in treating PSC.
Perioperative chemotherapy
Although perioperative chemotherapy has yielded improved rates of complete surgical resection, pathological remission, and survival in NSCLC, its efficacy in PSC remains underreported in prospective studies. A systematic review and meta-analysis indicated that neoadjuvant chemotherapy for NSCLC can reduce mortality by 13% (116). A retrospective study of PSC by Vieira et al. involved 20 patients (26%) who received neoadjuvant chemotherapy, with 55% of patients having PR, 40% with SD, and 5% with PD (17). A study from the Mayo Clinic also reported the benefits of neoadjuvant chemotherapy for patients with PSC (98).
Adjuvant therapy has become a standard treatment approach for some types of NSCLC (117), and cisplatin-based postoperative chemotherapy has been shown to improve OS in NSCLC (compared with the control group, the chemotherapy group showed a significant prolongation in OS (94 vs. 73 months; P=0.04), with 5-year survival rates of 69% and 54% respectively (P=0.03) (118,119). Surgery is the best treatment choice for patients with early-stage PSC. However, the high risk of postoperative recurrence suggests that adjuvant chemotherapy should be strongly considered. A meta-analysis suggested that patients receiving adjuvant chemotherapy have a significantly longer OS than those treated with surgery alone (120). Abdallah et al. compared 1,497 postoperative patients with PSC who received adjuvant chemotherapy with those who did not and found enhanced survival in stage II and III patients; however, stage III cases benefited in particular, with adjuvant chemotherapy resulting in a significant increase in OS from 12% to 30% (121). However, adjuvant chemotherapy was not found to be correlated with improved survival in stage I patients (121). Similarly, other studies suggest that adjuvant chemotherapy does not improve survival in patients with stage I PPC (122), while some have reported that adjuvant chemotherapy does not confer a survival benefit (99); this discrepancy may be due to the patients with different stages being included but not analyzed separately.
First-line chemotherapy demonstrates limited efficacy in PSC. However, perioperative chemotherapy has shown promise in improving survival for some patients (123). The mechanisms of PSC chemoresistance may be related to the sarcomatoid component (124) but remain underexamined, and thus chemotherapeutic strategies targeting the sarcomatoid component may be worth exploring. Overall, the chemotherapy-related treatment strategies in PSC need to be further investigated.
Radiotherapy
Radiotherapy serves as an important local therapeutic approach for treating patients with NSCLC across all clinical stages. Some studies have reported favorable outcomes among patients with PSC who have undergone radiotherapy (125,126). Gang et al. conducted a retrospective analysis of 1,039 patients with PSC using the SEER database and found that radiotherapy was an independent factor associated with a better prognosis for patients with PSC (127). Similarly, Xie et al. analyzed 400 patients with PSC and arrived to a similar conclusion (105). However, Rahouma et al. analyzed 4,987 patients with PSC and reported a median OS of 5 months for those receiving radiotherapy compared to 6 months for those without radiotherapy (P<0.001) (128). They also compared patients who received both neoadjuvant and adjuvant radiotherapy to those who received only adjuvant radiotherapy, with the median OS being 11 months and 9 months, respectively (128). In the study by Sun et al., patients who received adjuvant radiotherapy had a higher 5-year survival rate than did patients who did not receive treatment (55.4% vs. 29.4%; P<0.01) (129). Therefore, the efficacy of radiotherapy in PSC remains controversial, and this can be attributed to confounding variables within studies and the lack of subgroup analysis. Overall, perioperative radiotherapy appears to provide partial benefits to patients with PSC.
Targeted therapy
Targeted therapies mainly comprise small-molecule kinase inhibitors (SMKIs), which inhibit protein kinases involved in the biological processes of cancer cells, and monoclonal antibodies (mAbs), which work by targeting extracellular ligands and membrane receptors (130). The increasingly frequent discovery of novel therapeutic targets is carrying forth NSCLC treatment into the precision era. Given the high mutation frequency in PSC, the potential benefits of targeted therapy seem promising.
EGFR tyrosine kinase inhibitors (TKIs)
The significance of EGFR mutations in NSCLC has gained widespread recognition since they were first identified. The frequency of EGFR mutations in PSC is still controversial, with some studies suggesting that the frequency of EGFR mutations is as high as 20% in Asian populations (26,59), while it is lower in White populations (17,131-133). Types of EGFR mutations also vary across different studies. In contrast to the findings of Yang et al., which point to most EGFR mutations being common mutations (8), almost all EGFR mutations in the study by Fallet et al. were rare (88.9%) and were mainly concentrated in exons 2, 18, and 20, with the G719A mutation being the most common (55.5%) (7). More evidence is needed to clarify the role of EGFR mutations in PSC.
EGFR TKIs have proven to be effective as treatments for EGFR mutation-positive NSCLC, particularly in LUAD (gefitinib vs. chemotherapy, mPFS 10.8 vs. 5.4 months, P<0.001; erlotinib vs chemotherapy, mPFS 13.1 vs. 4.6 months, P<0.0001) (134,135). Nevertheless, the effectiveness of EGFR TKIs in PSC remains unclear. In some cases, the outcomes are unsatisfactory, and the clinical progression tends to be more rapid and occasionally accompanied by serious complications. In a case of advanced SCC with an EGFR exon 19 deletion mutation, the patient received gefitinib with no observable effect and died of respiratory failure 89 days later (136). Erlotinib was also unsatisfactory. A patient with advanced PPC with an EGFR exon 21 L858R mutation died just 1 week after starting erlotinib treatment due to rapid PD (137). Other patients have also not achieved long-term outcomes (138,139), but there are some reports in which EGFR TKIs demonstrated good efficacy (107,140).
The efficacy of EGFR TKIs in PSC remains to be further clarified, but poor efficacy appears to be reported for the majority of cases. Patients who do not respond to EGFR TKIs always tend to have rapid progression with severe complications. Caution should thus be exercised in the selection of treatment strategies for patients with PSC and EGFR mutations.
MET TKIs
MET mutations are more prevalent in PSC compared to other types of NSCLC (~3%), especially MET exon 14 skipping mutations (38,133,141,142). It has been widely demonstrated that MET TKIs benefit patients with NSCLC and MET exon 14 skipping mutations (a multicenter retrospective analysis evaluating the efficacy of MET TKIs indicates that NSCLC patients receiving treatment have a longer mOS compared to those who did not, 25.3 vs. 10.9 months) (143,144). Moreover, the efficacy of MET TKI in PSC has been demonstrated in prospective clinical studies.
A multicenter, single-arm, phase II clinical study (NCT02897479) evaluated the efficacy of the MET inhibitor savolitinib in patients with PSC and MET exon 14 skipping mutations. The study enrolled 70 patients, including 25 with PSC, treated with savolitinib once daily. The subgroup analysis indicated PR in 10 patients (ORR of 40.0%), a median duration of remission (DOR) of 17.9 months, and a median PFS of 5.5 months (97). Other clinical studies on MET TKI included only a limited number of patients with PSC. The phase 2 VISION trial reported an ORR of 46% for patients treated with tepotinib (145), while the phase 2 GEOMETRY mono-1 trial reported an ORR of 41% for camatinib in previously treated patients (146). Nonetheless, these trials included only a small fraction of patients with PSC (<8%) and lacked subgroup analyses. Case reports also point to the efficacy of crizotinib in patients with PSC and MET amplification (147,148).
Antiangiogenic therapy
Antiangiogenic therapy has become the cornerstone of second-line treatment or beyond for soft tissue sarcoma and the standard first-line treatment for some subtypes of soft tissue sarcoma. The presence of a sarcomatoid component in PSC suggests the potential effectiveness of antiangiogenic therapy.
Antiangiogenic monotherapy, such as that with apatinib or sorafenib, has shown favorable outcomes in case reports (149,150). Antiangiogenic combination therapy has also demonstrated promising results in PSC. For example, paclitaxel plus carboplatin combined with apatinib (151) or bevacizumab (152) both yielded benefits to patients. In one patient with SCC, complete response (CR) was sustained for 35 months after discontinuation of carboplatin plus paclitaxel and bevacizumab (153). A phase II study (UMIN000008707) included 9 patients treated with carboplatin plus paclitaxel alone or combination with bevacizumab. Patients who received combination therapy had an ORR of 44.4% (PR: 4; SD: 3; PD: 2) a median PFS of 4.2 months, and a median OS of 11.2 months, which was significantly superior to those who underwent chemotherapy alone (111). The feasibility of antiangiogenic therapy combined with chemotherapy is highly apparent. Interestingly, a patient with PPC was effectively and safely treated with bevacizumab plus paclitaxel for critical and refractory brain metastases after whole brain radiotherapy (WBRT), suggesting the possible value of radiotherapy combined with bevacizumab in patients with PSC (154). In some case reports, antiangiogenic therapy combined with immunotherapy provided promising results. A chemoresistant patient achieved CR over 20 months after treatment with tislelizumab plus anlotinib (109). Similarly, good efficacy was observed in cases treated with anlotinib combined with nivolumab (155), camrelizumab (156), or pembrolizumab (157).
Given the higher occurrence of angiogenesis in PSC, antiangiogenic therapy, particularly that combined with chemotherapy or immunotherapy, holds considerable promise. The presence of vascular invasion should also be considered during clinical treatment.
Immunotherapy
Immunotherapy mainly revolves around PD-1/PD-L1 and cytotoxic T lymphocyte–associated protein 4 (CTLA-4) inhibitors. PD-1/PD-L1 inhibitors have gained significant interest in the treatment of NSCLC. Their application has expanded from second-line to first-line treatments, from advanced to locally advanced and early stages, and from single-agent application to combination therapies. As previously mentioned, the high expression of PD-L1 and abundant immune infiltration in PSC suggest that immunotherapy will likely be effective in PSC (158). In a few reports, patients with PSC who failed first- and second-line chemotherapy demonstrated positive responses to immunotherapy (159,160).
Regarding the impact of first-line immunotherapy on patients with PSC, a multicenter retrospective study enrolled 21 patients with PSC receiving first-line immunotherapy (161). Of these patients, 14 received immunotherapy combined with anlotinib (tislelizumab: 7; camrelizumab: 4; sintilimab: 2; pembrolizumab: 1), 4 received immunotherapy combined with platinum-based chemotherapy (pembrolizumab: 2; sintilimab: 1; durvalumab: 1), and 3 patients received immune monotherapy (camrelizumab: 2; sintilimab: 1) (161). The ORR was 57.1%, the disease control rate (DCR) was 81% (PR: 12; SD: 5; PD: 4), the median PFS was 9.2 months, and the median OS was 22.8 months (161). Immune monotherapy, immunotherapy combined with anlotinib, and immunotherapy combined with chemotherapy yielded a median PFS of 8.0, 9.4, and 9.6 months, respectively, while the median OS was 19.0, 22.8, and 30.6 months, respectively (161). No statistical differences in PFS or OS were observed between treatment strategies or different ICIs (161). Wei et al. reviewed 33 patients who received immune monotherapy (8 patients) or immune combination therapy (25 patients; immunotherapy combined with chemotherapy or targeted therapy) as first-line (19 patients) or second-line (14 patients) treatment. Among all patients, the ORR was 36.4% and the DCR was 78.8%. The median PFS and OS were 6.07 and 21.33 months, respectively (162). Similarly, subgroup analysis indicated no statistically significant differences in ORR, DCR, PFS, or OS across treatment modalities (162).
Efficacy has also been demonstrated in patients treated with second-line therapy or beyond. A multicenter retrospective study by Domblides et al. included 37 patients with PSC treated with ICIs as second-line treatment (20 patients) or beyond (17 patients) who had received first-line platinum-based chemotherapy before immunotherapy. Of these patients, 32 patients received nivolumab, 3 received pembrolizumab, and 2 received atezolizumab (37). The ORR was 40.5%, the DCR was 64.8%, the median PFS was 4.89 months, and the OS was 12.7 months (37). The ORR of patients with PSC was twice as high as that of those with other types of NSCLC (40.5% vs. 20%) (37,163). Another similar study involving 49 patients who received pembrolizumab (40 patients), nivolumab (7 patients), or atezolizumab (2 patients), with 38 on second-line therapy, reported an ORR of 49.0%, a median PFS of 7.2 months, and a median OS of 22.2 months (164).
Regarding dual immunotherapy, a phase II study (NCT03022500) evaluated the efficacy and safety of durvalumab plus tremelimumab in recurrent or metastatic PSC (41). Out of 18 enrolled patients, 15 were analyzed for the primary endpoint. The ORR was 26.7%, and the median PFS and OS were 5.9 and 15.4 months, respectively (41).
Both first- and second-line immunotherapy and immune-combination therapy have demonstrated reliable efficacy in PSC. However, there have been reports of severe adverse reactions or rapid progression following immunotherapy in some cases (37,165), emphasizing the importance of comprehensive consideration in clinical decision-making. Most recent or ongoing clinical studies in PSC are focused on immunotherapy (Table 2).
Table 2
| Trial number | Study type | Study phase | Therapeutic agent | Primary outcome | Start of enrollment | Study status |
|---|---|---|---|---|---|---|
| NCT04888429 | Interventional | Phase II | Camrelizumab + famitinib | ORR | 2021/7/19 | Recruiting |
| NCT04725448 | Interventional | Phase II | Toripalimab + bevacizumab + nab-paclitaxel + carboplatin | PFS | 2021/4/6 | Recruiting |
| NCT04224337 | Interventional | Phase II | Durvalumab + doxorubicin + ifosfamide | RR | 2020/6/11 | – |
| UMIN000027629 | Interventional | Phase II | Pembrolizumab | OR | 2017/6/12 | No longer recruiting |
| NCT03022500 | Interventional | Phase II | Durvalumab + tremelimumab | RR | 2017/5/18 | – |
| NCT02834013 | Interventional | Phase II | Ipilimumab + nivolumab | ORR | 2017/1/13 | Active, not recruiting |
| NCT02897479 | Interventional | Phase II | Savolitinib | ORR | 2016/12/1 | – |
| UMIN000023433 | Interventional | Phase II | Nivolumab | OR | 2016/11/1 | No longer recruiting |
| UMIN000008707 | Interventional | Phase II | Carboplatin + paclitaxel + bevacizumab/carboplatin + paclitaxel | RR | 2012/8/17 | – |
| NCT05337163 | Observational | – | – | – | 2022/2/25 | Recruiting |
| NCT04215913 | Observational | – | – | – | 2019/12/30 | Not yet recruiting |
PSC, pulmonary sarcomatoid carcinoma; ORR, objective response rate; PFS, progression-free survival; RR, response rate; OR, overall response.
Conclusions
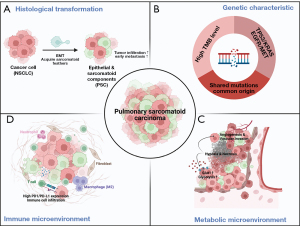
The complexity of PSC as a highly malignant subgroup of NSCLC is compounded by its histologic subtypes and diverse cellular compositions. Although many studies (8,11-13,25-35) have confirmed the common origin of PSC’s two components, in vitro and in vivo research has not sufficiently advanced to replicate this translational process. However, achieving this requires a comprehensive understanding of the cellular status of the sarcomatoid component, for which techniques such as single-cell RNA sequencing, spatial omics, or proteomics may be useful. PSC displays a substantially high degree of genetic mutation although the frequency and types of mutations vary across different ethnicities, indicating significant heterogeneity among patients. Therefore, genetic testing is necessary for the precise treatment decision. High levels of hypoxia, glycolysis, and angiogenesis with vascular invasion might contribute to drug resistance and frequent metastasis in PSC, with this being potentially linked to the specific microenvironment of the sarcomatoid component. However, these correlations need to be validated by basic research. The high expression of immune checkpoints and the infiltration of immune cells suggest that PSC can be classified as a hot tumor with adaptive immune resistance. The various biological characteristics of PSC are summarized in Figure 2.
The unfavorable prognosis of PSC can be attributed to its resistance to chemotherapy and radiotherapy. A higher mutation frequency and immune infiltration suggest that targeted therapy and immunotherapy may be highly efficacious for PSC (Figure 3). Savolitinib is the only MET inhibitor that has been prospectively studied in patients with MET exon 14 skipping mutations. There are various opinions concerning the efficacy of inhibitors targeting other actionable mutation sites, necessitating systematic prospective clinical validation. The potential of antiangiogenic therapy in PSC appears promising, and clinical study indicate that antiangiogenic therapy combined with chemotherapy is significantly more effective than chemotherapy alone (111). Retrospective analyses of first- and second-line immunotherapies have yielded favorable outcomes, and dual immunotherapy trials have also shown promise, as have combination therapies. Various dual antibodies such as PD-1 plus VEGF or PD-L1 plus VEGF are also worth attempting in PSC.
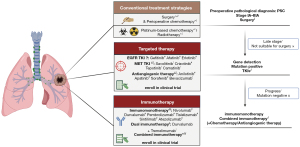
To date, most of the literature in PSC consists of retrospective studies and case reports. Prospective clinical data and basic biological studies for PSC are limited. Multiple centers worldwide are engaging in prospective clinical studies evaluating a variety of treatment strategies for PSC (Table 2). Basic research in multiomics related to PSC should also be undertaken.
Acknowledgments
We would like to acknowledge the support of Tongji Hospital, Huazhong University of Science and Technology and Xinqiao Hospital, Third Military Medical University for this work.
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-127/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-127/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-127/coif). Z.J. is from GloriousMed Clinical Laboratory (Shanghai) Co., Ltd., Shanghai, China. L.B. received grants or contracts from Takeda, Roche, AstraZeneca, and BMS; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Invitae, Eli-Lilly, AstraZeneca, Roche, MSD, Merck, BMS, Pfizer, Novartis, Takeda, Janssen, and Daiichi Sankyo; and support for attending meetings from Pfizer; and participated in advisory boards of Invitae, Eli-Lilly, AstraZeneca, Roche, MSD, Merck, BMS, Pfizer, Novartis, Takeda, and Janssen; and is currently member of Int. Secretary- Austrian Society of Pathology; member of PPS Membership and Awards Committee; member of the Rare Cancers Committee of the IASLC. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery 2012;152:397-402. [Crossref] [PubMed]
- Ung M, Rouquette I, Filleron T, et al. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin Lung Cancer 2016;17:391-7. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013;8:1574-7. [Crossref] [PubMed]
- Jordan EJ, Kim HR, Arcila ME, et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov 2017;7:596-609. [Crossref] [PubMed]
- Lococo F, Gandolfi G, Rossi G, et al. Deep Sequencing Analysis Reveals That KRAS Mutation Is a Marker of Poor Prognosis in Patients with Pulmonary Sarcomatoid Carcinoma. J Thorac Oncol 2016;11:1282-92. [Crossref] [PubMed]
- Fallet V, Saffroy R, Girard N, et al. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCarta Panel: exploring therapeutic targets. Ann Oncol 2015;26:1748-53. [Crossref] [PubMed]
- Yang Z, Xu J, Li L, et al. Integrated molecular characterization reveals potential therapeutic strategies for pulmonary sarcomatoid carcinoma. Nat Commun 2020;11:4878. [Crossref] [PubMed]
- Pang A, Carbini M, Moreira AL, et al. Carcinosarcomas and Related Cancers: Tumors Caught in the Act of Epithelial-Mesenchymal Transition. J Clin Oncol 2018;36:210-6. [Crossref] [PubMed]
- Matsui K, Kitagawa M. Spindle cell carcinoma of the lung. A clinicopathologic study of three cases. Cancer 1991;67:2361-7. [Crossref] [PubMed]
- Yang Z, Shen Y, Jiang J, et al. Transformation of Two Cases of Lung Adenocarcinoma into Pulmonary Sarcomatoid Carcinoma following Treatment. Can Respir J 2021;2021:6661772. [Crossref] [PubMed]
- Xie X, Chen X, Luo N, et al. Transformation of invasive lung adenocarcinoma with ALK rearrangement into pulmonary sarcomatoid carcinoma. J Cancer Res Clin Oncol 2022;148:2165-8. [Crossref] [PubMed]
- Arshi J, Sauer M, Yin F. Rapid Sarcomatoid Transformation of Lung Squamous Cell Carcinoma After Neoadjuvant Therapy: A Case Report. Anticancer Res 2020;40:1625-9. [Crossref] [PubMed]
- Saito T, Tsuta K, Fukumoto KJ, et al. Combined small cell lung carcinoma and giant cell carcinoma: a case report. Surg Case Rep 2017;3:52. [Crossref] [PubMed]
- Iezumi K, Masunaga A, Kadofuku T, et al. Combined small cell carcinoma with pulmonary blastoma and adenocarcinoma: case report and clonality analysis. Pathol Res Pract 2006;202:895-9. [Crossref] [PubMed]
- Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003;27:311-24. [Crossref] [PubMed]
- Vieira T, Antoine M, Ruppert AM, et al. Blood vessel invasion is a major feature and a factor of poor prognosis in sarcomatoid carcinoma of the lung. Lung Cancer 2014;85:276-81. [Crossref] [PubMed]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003;112:1776-84. [Crossref] [PubMed]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006;7:131-42. [Crossref] [PubMed]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392-401. [Crossref] [PubMed]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8-20. [Crossref] [PubMed]
- Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol 2010;18:103-20. [Crossref] [PubMed]
- Huszar M, Herczeg E, Lieberman Y, et al. Distinctive immunofluorescent labeling of epithelial and mesenchymal elements of carcinosarcoma with antibodies specific for different intermediate filaments. Hum Pathol 1984;15:532-8. [Crossref] [PubMed]
- Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol 1996;20:277-85. [Crossref] [PubMed]
- Zidar N, Gale N. Carcinosarcoma and spindle cell carcinoma--monoclonal neoplasms undergoing epithelial-mesenchymal transition. Virchows Arch 2015;466:357-8. [Crossref] [PubMed]
- Chang YL, Wu CT, Shih JY, et al. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol 2011;18:2952-60. [Crossref] [PubMed]
- Takahashi K, Kohno T, Matsumoto S, et al. Clonality and heterogeneity of pulmonary blastoma from the viewpoint of genetic alterations: a case report. Lung Cancer 2007;57:103-8. [Crossref] [PubMed]
- Manzotti G, Torricelli F, Benedetta D, et al. An Epithelial-to-Mesenchymal Transcriptional Switch Triggers Evolution of Pulmonary Sarcomatoid Carcinoma (PSC) and Identifies Dasatinib as New Therapeutic Option. Clin Cancer Res 2019;25:2348-60. [Crossref] [PubMed]
- Liu T, Zhao X, Zheng X, et al. The EMT transcription factor, Twist1, as a novel therapeutic target for pulmonary sarcomatoid carcinomas. Int J Oncol 2020;56:750-60. [Crossref] [PubMed]
- Blaukovitsch M, Halbwedl I, Kothmaier H, et al. Sarcomatoid carcinomas of the lung--are these histogenetically heterogeneous tumors? Virchows Arch 2006;449:455-61. [Crossref] [PubMed]
- Miyahara S, Hamasaki M, Hamatake D, et al. Clinicopathological analysis of pleomorphic carcinoma of the lung: diffuse ZEB1 expression predicts poor survival. Lung Cancer 2015;87:39-44. [Crossref] [PubMed]
- Tamaki T, Shimizu T, Niki M, et al. Immunohistochemical analysis of NANOG expression and epithelial-mesenchymal transition in pulmonary sarcomatoid carcinoma. Oncol Lett 2017;13:3695-702. [Crossref] [PubMed]
- Viswanathan K, Siddiqui MT, Borczuk AC. The diagnostic utility of zinc E-box 1 (ZEB1) transcription factor for identification of pulmonary sarcomatoid carcinoma in cytologic and surgical specimens. J Am Soc Cytopathol 2020;9:55-61. [Crossref] [PubMed]
- Pelosi G, Melotti F, Cavazza A, et al. A modified vimentin histological score helps recognize pulmonary sarcomatoid carcinoma in small biopsy samples. Anticancer Res 2012;32:1463-73.
- Shimizu S, Sakai K, Chikugo T, et al. Integrin-linked kinase pathway in heterogeneous pulmonary sarcomatoid carcinoma. Oncol Lett 2021;21:320. [Crossref] [PubMed]
- Ullah A, Ahmed A, Yasinzai AQK, et al. Demographics and Clinicopathologic Profile of Pulmonary Sarcomatoid Carcinoma with Survival Analysis and Genomic Landscape. Cancers (Basel) 2023;15:2469. [Crossref] [PubMed]
- Domblides C, Leroy K, Monnet I, et al. Efficacy of Immune Checkpoint Inhibitors in Lung Sarcomatoid Carcinoma. J Thorac Oncol 2020;15:860-6. [Crossref] [PubMed]
- Schrock AB, Li SD, Frampton GM, et al. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J Thorac Oncol 2017;12:932-42. [Crossref] [PubMed]
- Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol 2016;34:794-802. [Crossref] [PubMed]
- Jiang X, Liu Y, Chen C, et al. The value of biomarkers in patients with sarcomatoid carcinoma of the lung: molecular analysis of 33 cases. Clin Lung Cancer 2012;13:288-96. [Crossref] [PubMed]
- Kim M, Keam B, Ock CY, et al. Phase II study of durvalumab and tremelimumab in pulmonary sarcomatoid carcinoma: KCSG-LU16-07. Thorac Cancer 2020;11:3482-9. [Crossref] [PubMed]
- Li X, Wang D, Zhao Q, et al. Clinical Significance and Next-Generation Sequencing of Chinese Pulmonary Sarcomatoid Carcinoma. Sci Rep 2017;7:3947. [Crossref] [PubMed]
- Yu Y, Duan X, Wang S, et al. Analysis of molecular pathologic and clinical features of 36 patients with pulmonary sarcomatoid carcinoma. BMC Pulm Med 2022;22:453. [Crossref] [PubMed]
- Forest F, Yvorel V, Karpathiou G, et al. Histomolecular profiling of pleomorphic, spindle cell, and giant cell carcinoma of the lung for targeted therapies. Hum Pathol 2016;49:99-106. [Crossref] [PubMed]
- Liu X, Wang F, Xu C, et al. Genomic origin and intratumor heterogeneity revealed by sequencing on carcinomatous and sarcomatous components of pulmonary sarcomatoid carcinoma. Oncogene 2021;40:821-32. [Crossref] [PubMed]
- Pécuchet N, Vieira T, Rabbe N, et al. Molecular classification of pulmonary sarcomatoid carcinomas suggests new therapeutic opportunities. Ann Oncol 2017;28:1597-604. [Crossref] [PubMed]
- Jing X, Yang F, Shao C, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer 2019;18:157. [Crossref] [PubMed]
- McDonald PC, Chafe SC, Dedhar S. Overcoming Hypoxia-Mediated Tumor Progression: Combinatorial Approaches Targeting pH Regulation, Angiogenesis and Immune Dysfunction. Front Cell Dev Biol 2016;4:27. [Crossref] [PubMed]
- Qiu GZ, Jin MZ, Dai JX, et al. Reprogramming of the Tumor in the Hypoxic Niche: The Emerging Concept and Associated Therapeutic Strategies. Trends Pharmacol Sci 2017;38:669-86. [Crossref] [PubMed]
- Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int 2015;2015:549412. [Crossref] [PubMed]
- Tsubata Y, Sutani A, Okimoto T, et al. Comparative analysis of tumor angiogenesis and clinical features of 55 cases of pleomorphic carcinoma and adenocarcinoma of the lung. Anticancer Res 2015;35:389-94.
- Saito S, Lin YC, Tsai MH, et al. Emerging roles of hypoxia-inducible factors and reactive oxygen species in cancer and pluripotent stem cells. Kaohsiung J Med Sci 2015;31:279-86. [Crossref] [PubMed]
- Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 2008;15:678-85. [Crossref] [PubMed]
- Chang YL, Yang CY, Lin MW, et al. High co-expression of PD-L1 and HIF-1alpha correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer 2016;60:125-35. [Crossref] [PubMed]
- Mochizuki T, Ishii G, Nagai K, et al. Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am J Surg Pathol 2008;32:1727-35. [Crossref] [PubMed]
- Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer 2021;21:669-80. [Crossref] [PubMed]
- Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 2007;12:108-13. [Crossref] [PubMed]
- Ito K, Oizumi S, Fukumoto S, et al. Clinical characteristics of pleomorphic carcinoma of the lung. Lung Cancer 2010;68:204-10. [Crossref] [PubMed]
- Kaira K, Horie Y, Ayabe E, et al. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol 2010;5:460-5. [Crossref] [PubMed]
- Kaira K, Endo M, Abe M, et al. Biologic correlates of (1)(8)F-FDG uptake on PET in pulmonary pleomorphic carcinoma. Lung Cancer 2011;71:144-50. [Crossref] [PubMed]
- Rapicetta C, Lococo F, Stefani A, et al. Primary Sarcomatoid Carcinoma of the Lung: Radiometabolic ((18)F-FDG PET/CT) Findings and Correlation with Clinico-Pathological and Survival Results. Lung 2016;194:653-7. [Crossref] [PubMed]
- Isselbacher KJ. Sugar and amino acid transport by cells in culture--differences between normal and malignant cells. N Engl J Med 1972;286:929-33. [Crossref] [PubMed]
- Yamamoto T, Seino Y, Fukumoto H, et al. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun 1990;170:223-30. [Crossref] [PubMed]
- Younes M, Brown RW, Stephenson M, et al. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer 1997;80:1046-51. [Crossref] [PubMed]
- Higashi K, Ueda Y, Sakurai A, et al. Correlation of Glut-1 glucose transporter expression with. Eur J Nucl Med 2000;27:1778-85.
- Chung JH, Cho KJ, Lee SS, et al. Overexpression of Glut1 in lymphoid follicles correlates with false-positive (18)F-FDG PET results in lung cancer staging. J Nucl Med 2004;45:999-1003.
- Kaira K, Oriuchi N, Otani Y, et al. Fluorine-18-alpha-methyltyrosine positron emission tomography for diagnosis and staging of lung cancer: a clinicopathologic study. Clin Cancer Res 2007;13:6369-78. [Crossref] [PubMed]
- Imai H, Kaira K, Endoh H, et al. Prognostic Significance of Glucose Metabolism as GLUT1 in Patients with Pulmonary Pleomorphic Carcinoma. J Clin Med 2020;9:413. [Crossref] [PubMed]
- Kawano R, Takeshima Y, Inai K. Alteration of the p53 gene of lung carcinomas with sarcomatous transformation (spindle cell carcinoma): analysis of four cases. Pathol Int 1996;46:38-45. [Crossref] [PubMed]
- Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res 1974;34:997-1004.
- Rigau V, Molina TJ, Chaffaud C, et al. Blood vessel invasion in resected non small cell lung carcinomas is predictive of metastatic occurrence. Lung Cancer 2002;38:169-76. [Crossref] [PubMed]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [Crossref] [PubMed]
- Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol 2001;2:667-73. [Crossref] [PubMed]
- Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol 2009;19:329-37. [Crossref] [PubMed]
- Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol 2006;76:217-57. [Crossref] [PubMed]
- Meert AP, Paesmans M, Martin B, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2002;87:694-701. [Crossref] [PubMed]
- Macchiarini P, Fontanini G, Hardin MJ, et al. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet 1992;340:145-6. [Crossref] [PubMed]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer 2002;2:38-47. [Crossref] [PubMed]
- Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001;85:881-90. [Crossref] [PubMed]
- Kaseda K, Ishii G, Aokage K, et al. Identification of intravascular tumor microenvironment features predicting the recurrence of pathological stage I lung adenocarcinoma. Cancer Sci 2013;104:1262-9. [Crossref] [PubMed]
- Wang J, Chen J, Chen X, et al. Blood vessel invasion as a strong independent prognostic indicator in non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2011;6:e28844. [Crossref] [PubMed]
- Yuki T, Sakuma T, Ohbayashi C, et al. Pleomorphic carcinoma of the lung: a surgical outcome. J Thorac Cardiovasc Surg 2007;134:399-404. [Crossref] [PubMed]
- Okuda K, Oda R, Suzuki A, et al. Clinicopathological factors influenced the prognosis of surgically resected pulmonary pleomorphic carcinoma. J Thorac Dis 2017;9:1295-302. [Crossref] [PubMed]
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol 2013;8:803-5. [Crossref] [PubMed]
- Wang F, Cali Daylan AE, Deng L, et al. Heterogeneous Expression of PD-L1, B7x, B7-H3, and HHLA2 in Pulmonary Sarcomatoid Carcinoma and the Related Regulatory Signaling Pathways. Cancers (Basel) 2023;15:3372. [Crossref] [PubMed]
- Sim JK, Chung SM, Choi JH, et al. Clinical and molecular characteristics of pulmonary sarcomatoid carcinoma. Korean J Intern Med 2018;33:737-44. [Crossref] [PubMed]
- Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698-707. [Crossref] [PubMed]
- Nagano M, Kohsaka S, Hayashi T, et al. Comprehensive molecular profiling of pulmonary pleomorphic carcinoma. NPJ Precis Oncol 2021;5:57. [Crossref] [PubMed]
- Imanishi N, Hirai A, Yoneda K, et al. Programmed death-ligand 1 (PD-L1) expression in pleomorphic carcinoma of the lung. J Surg Oncol 2018;117:1563-9. [Crossref] [PubMed]
- Zhou F, Guo H, Zhou X, et al. Immune checkpoint inhibitors plus chemotherapy in patients with locally advanced or metastatic pulmonary sarcomatoid carcinoma: a multicentric real-world study. Ther Adv Med Oncol 2022;14:17588359221136759. [Crossref] [PubMed]
- Vieira T, Antoine M, Hamard C, et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016;98:51-8. [Crossref] [PubMed]
- Ma Y, Li W, Li Z, et al. Immunophenotyping of pulmonary sarcomatoid carcinoma. Front Immunol 2022;13:976739. [Crossref] [PubMed]
- Zhang C, Li Z, Zhang Y, et al. Genomic Variations and Immune-Related Features of TMB, PD-L1 Expression and CD8(+) T Cell Infiltration in Chinese Pulmonary Sarcomatoid Carcinoma. Int J Gen Med 2022;15:4209-20. [Crossref] [PubMed]
- Chen J, He Q, Liu J, et al. CD8+ tumor-infiltrating lymphocytes as a novel prognostic biomarker in lung sarcomatoid carcinoma, a rare subtype of lung cancer. Cancer Manag Res 2018;10:3505-11. [Crossref] [PubMed]
- Amemiya R, Miyoshi T, Aokage K, et al. Prognostic impact of the tumor immune microenvironment in pulmonary pleomorphic carcinoma. Lung Cancer 2021;153:56-65. [Crossref] [PubMed]
- Seong YW, Han SJ, Jung W, et al. Perioperative change in neutrophil-to-lymphocyte ratio (NLR) is a prognostic factor in patients with completely resected primary pulmonary sarcomatoid carcinoma. J Thorac Dis 2019;11:819-26. [Crossref] [PubMed]
- Lu S, Fang J, Li X, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med 2021;9:1154-64. [Crossref] [PubMed]
- Maneenil K, Xue Z, Liu M, et al. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin Lung Cancer 2018;19:e323-33. [Crossref] [PubMed]
- Gong T, Jia B, Chen C, et al. Clinical analysis of 78 pulmonary sarcomatoid carcinomas with surgical treatment. J Int Med Res 2022;50:3000605221128092. [Crossref] [PubMed]
- Chang YL, Lee YC, Shih JY, et al. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer 2001;34:91-7. [Crossref] [PubMed]
- Kim TS, Han J, Lee KS, et al. CT findings of surgically resected pleomorphic carcinoma of the lung in 30 patients. AJR Am J Roentgenol 2005;185:120-5. [Crossref] [PubMed]
- Mignard X, Ruppert AM, Antoine M, et al. c-MET Overexpression as a Poor Predictor of MET Amplifications or Exon 14 Mutations in Lung Sarcomatoid Carcinomas. J Thorac Oncol 2018;13:1962-7. [Crossref] [PubMed]
- Ferhatoglu F, Amirov F, Ozkan B, et al. Clinicopathological and Prognostic Features of 67 Cases with Pulmonary Sarcomatoid Carcinoma: An 18-Year Single-Centre Experience. Oncol Res Treat 2021;44:590-601. [Crossref] [PubMed]
- Zeng Q, Li J, Sun N, et al. Preoperative systemic immune-inflammation index predicts survival and recurrence in patients with resected primary pulmonary sarcomatoid carcinoma. Transl Lung Cancer Res 2021;10:18-31. [Crossref] [PubMed]
- Xie Y, Lin Z, Shi H, et al. The Prognosis of Pulmonary Sarcomatoid Carcinoma: Development and Validation of a Nomogram Based on SEER. Technol Cancer Res Treat 2022;21:15330338221109647. [Crossref] [PubMed]
- Lococo F, Rapicetta C, Cardillo G, et al. Pathologic Findings and Long-Term Results After Surgical Treatment for Pulmonary Sarcomatoid Tumors: A Multicenter Analysis. Ann Thorac Surg 2017;103:1142-50. [Crossref] [PubMed]
- Saitoh M, Niijima M, Takiguchi Y, et al. An early event of EGFR mutation in pleomorphic carcinoma of the lung. Int J Clin Oncol 2011;16:770-3. [Crossref] [PubMed]
- Nishioka N, Kaneko Y, Yamada T, et al. Effective combined therapy with ramucirumab for advanced pulmonary pleomorphic carcinoma. Respirol Case Rep 2018;6:e00372. [Crossref] [PubMed]
- Li YF, Zhao XF, Tian Y, et al. Case Report: Pulmonary sarcomatoid carcinoma complicating TP53 mutation treated successfully with Tislelizumab combined with Anlotinib-a case report. Front Genet 2022;13:949989. [Crossref] [PubMed]
- Lara PN Jr, Redman MW, Kelly K, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol 2008;26:463-7. [Crossref] [PubMed]
- Oizumi S, Takamura K, Harada T, et al. Phase II study of carboplatin-paclitaxel alone or with bevacizumab in advanced sarcomatoid carcinoma of the lung: HOT1201/NEJ024. Int J Clin Oncol 2022;27:676-83. [Crossref] [PubMed]
- Ogura K, Goto T, Imanishi J, et al. Neoadjuvant and adjuvant chemotherapy with modified mesna, adriamycin, ifosfamide, and dacarbazine (MAID) regimen for adult high-grade non-small round cell soft tissue sarcomas. Int J Clin Oncol 2013;18:170-6. [Crossref] [PubMed]
- Fayette J, Penel N, Chevreau C, et al. Phase III trial of standard versus dose-intensified doxorubicin, ifosfamide and dacarbazine (MAID) in the first-line treatment of metastatic and locally advanced soft tissue sarcoma. Invest New Drugs 2009;27:482-9. [Crossref] [PubMed]
- Lee KW, Kim YJ, Kim JH, et al. Two consecutive cases of platinum-refractory pulmonary pleomorphic carcinoma that showed dramatic responses to MAID (mesna, doxorubicin, ifosfamide and dacarbazine) chemotherapy. Jpn J Clin Oncol 2011;41:430-3. [Crossref] [PubMed]
- Lee J, Jung HA, Kim Y, et al. Efficacy of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) in patients with advanced pulmonary pleomorphic carcinoma. Lung Cancer 2018;122:160-4. [Crossref] [PubMed]
- Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Zombori-Tóth N, Kiss S, Oštarijaš E, et al. Adjuvant chemotherapy could improve the survival of pulmonary sarcomatoid carcinoma: A systematic review and meta-analysis. Surg Oncol 2022;44:101824. [Crossref] [PubMed]
- Abdallah HM, Martinez-Meehan D, Lutfi W, et al. Adjuvant chemotherapy for pulmonary sarcomatoid carcinoma: A retrospective analysis of the National Cancer Database. J Thorac Cardiovasc Surg 2022;163:1669-1681.e3. [Crossref] [PubMed]
- Hendriksen BS, Hollenbeak CS, Reed MF, et al. Perioperative chemotherapy is not associated with improved survival in stage I pleomorphic lung cancer. J Thorac Cardiovasc Surg 2019;158:581-591.e11. [Crossref] [PubMed]
- Chaft JE, Sima CS, Ginsberg MS, et al. Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung. J Thorac Oncol 2012;7:1400-5. [Crossref] [PubMed]
- Giroux Leprieur E, Antoine M, Vieira T, et al. Clinical and molecular features in patients with advanced non-small-cell lung carcinoma refractory to first-line platinum-based chemotherapy. Lung Cancer 2013;79:167-72. [Crossref] [PubMed]
- Awobajo MD, Vaporciyan AA, Lu C, et al. Stereotactic body radiation therapy (SBRT) in the management of pulmonary spindle cell carcinoma. BMJ Case Rep 2020;13:e234779. [Crossref] [PubMed]
- Yaguchi D, Ichikawa M, Ito M, et al. Dramatic response to nivolumab after local radiotherapy in pulmonary pleomorphic carcinoma with rapid progressive post-surgical recurrence. Thorac Cancer 2019;10:1263-6. [Crossref] [PubMed]
- Gang J, Yan Q, Xiang S, et al. Clinicopathological characteristics and prognostic factors of pulmonary sarcomatoid carcinoma: a large population analysis. Ann Transl Med 2021;9:121. [Crossref] [PubMed]
- Rahouma M, Kamel M, Narula N, et al. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the Surveillance, Epidemiology, and End Results database. Eur J Cardiothorac Surg 2018;53:828-34. [Crossref] [PubMed]
- Sun L, Dai J, Chen Y, et al. Pulmonary Sarcomatoid Carcinoma: Experience From SEER Database and Shanghai Pulmonary Hospital. Ann Thorac Surg 2020;110:406-13. [Crossref] [PubMed]
- Min HY, Lee HY. Molecular targeted therapy for anticancer treatment. Exp Mol Med 2022;54:1670-94. [Crossref] [PubMed]
- Pelosi G, Gasparini P, Cavazza A, et al. Multiparametric molecular characterization of pulmonary sarcomatoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer 2012;77:507-14. [Crossref] [PubMed]
- Italiano A, Cortot AB, Ilie M, et al. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-EGFR treatment of a rare lung malignancy. Int J Cancer 2009;125:2479-82. [Crossref] [PubMed]
- Stephan-Falkenau S, Streubel A, Mairinger T, et al. Integrated Clinical, Molecular and Immunological Characterization of Pulmonary Sarcomatoid Carcinomas Reveals an Immune Escape Mechanism That May Influence Therapeutic Strategies. Int J Mol Sci 2023;24:10558. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Ikushima H, Sakatani T, Masuda Y, et al. Lung spindle cell carcinoma harbouring a constitutively active epidermal growth factor receptor mutation. Respirol Case Rep 2019;7:e00395. [Crossref] [PubMed]
- Masuda K, Tokito T, Azuma K, et al. Pulmonary pleomorphic carcinoma: A case harboring EGFR mutation treated with EGFR-TKIs. Thorac Cancer 2018;9:754-7. [Crossref] [PubMed]
- Xia L, Shao YW, Xia Y. Nkx2-4 Mutation Confers Resistance to EGFR-Tyrosine Kinase Inhibitors in EGFR-Mutant Lung Sarcomatoid Carcinoma. J Thorac Oncol 2019;14:e125-6. [Crossref] [PubMed]
- Oguri T, Sasada S, Seki S, et al. A case of hyperprogressive disease following atezolizumab therapy for pulmonary pleomorphic carcinoma with epidermal growth factor receptor mutation. Respir Med Case Rep 2021;33:101405. [Crossref] [PubMed]
- Nakamura D, Miura K, Kumeda H, et al. Successful Resection of G719X-Positive Pleomorphic Carcinoma after Afatinib Treatment. Case Rep Oncol 2017;10:1035-40. [Crossref] [PubMed]
- Vuong HG, Ho ATN, Altibi AMA, et al. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer - A systematic review and meta-analysis. Lung Cancer 2018;123:76-82. [Crossref] [PubMed]
- Gow CH, Hsieh MS, Chen YL, et al. Survival outcomes and prognostic factors of lung cancer patients with the MET exon 14 skipping mutation: A single-center real-world study. Front Oncol 2023;13:1113696. [Crossref] [PubMed]
- Salgia R, Sattler M, Scheele J, et al. The promise of selective MET inhibitors in non-small cell lung cancer with MET exon 14 skipping. Cancer Treat Rev 2020;87:102022. [Crossref] [PubMed]
- Miranda O, Farooqui M, Siegfried JM. Status of Agents Targeting the HGF/c-Met Axis in Lung Cancer. Cancers (Basel) 2018;10:280. [Crossref] [PubMed]
- Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 2020;383:931-43. [Crossref] [PubMed]
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- He Q, Shi X, Zhu H, et al. A case treated with Crizotinib after secondary MET amplification of A double Rare L747S and G719S EGFR mutation Pulmonary Sarcomatoid Carcinoma. Ann Oncol 2020;31:544-6. [Crossref] [PubMed]
- Wang X, Cao J, Du W, et al. Response to gefitinib/crizotinib combination in a pulmonary sarcomatoid carcinoma patient harboring concurrent EGFR mutation and MET amplification. Clin Case Rep 2021;9:e04487. [Crossref] [PubMed]
- Li X, He Y, Zhu J, et al. Apatinib-based targeted therapy against pulmonary sarcomatoid carcinoma: a case report and literature review. Oncotarget 2018;9:33734-8. [Crossref] [PubMed]
- Mulamalla K, Truskinovsky AM, Dudek AZ. Pulmonary blastoma with renal metastasis responds to sorafenib. J Thorac Oncol 2007;2:344-7. [Crossref] [PubMed]
- Kong FW, Wang WM, Liu L, et al. First-line albumin-bound paclitaxel/carboplatin plus apatinib in advanced pulmonary sarcomatoid carcinoma: A case series and review of the literature. Medicine (Baltimore) 2020;99:e20667. [Crossref] [PubMed]
- Oda T, Sekine A, Kato T, et al. Promising effect of chemotherapy with bevacizumab for patients with pulmonary pleomorphic carcinoma: Two case reports and a literature review. Respir Investig 2015;53:296-9. [Crossref] [PubMed]
- Sakata S, Saeki S, Sato R, et al. Long-term complete response to carboplatin plus paclitaxel combined with bevacizumab in a patient with metastatic spindle cell carcinoma. Respir Investig 2017;55:372-5. [Crossref] [PubMed]
- Hata A, Fujita S, Takayama K, et al. Bevacizumab for critical brain metastases in a patient with pulmonary pleomorphic carcinoma. Intern Med 2014;53:1813-8. [Crossref] [PubMed]
- Jin C, Yang B. Dramatic Response of Pulmonary Sarcomatoid Carcinoma to Nivolumab Combined with Anlotinib: A Case Report. Case Rep Oncol 2020;13:601-5. [Crossref] [PubMed]
- Luo Y, Wei J, Zhang J, et al. Two different patients with pulmonary pleomorphic carcinoma response to PD-1 inhibitor plus anlotinib. Lung Cancer 2021;155:170-4. [Crossref] [PubMed]
- Wu S, Wu S, Liao X, et al. Pembrolizumab combined with anlotinib improves therapeutic efficacy in pulmonary sarcomatoid carcinoma with TMB-H and PD-L1 expression: a case report and literature review. Front Immunol 2023;14:1274937. [Crossref] [PubMed]
- Zhou F, Huang Y, Cai W, et al. The genomic and immunologic profiles of pure pulmonary sarcomatoid carcinoma in Chinese patients. Lung Cancer 2021;153:66-72. [Crossref] [PubMed]
- Salati M, Baldessari C, Calabrese F, et al. Nivolumab-Induced Impressive Response of Refractory Pulmonary Sarcomatoid Carcinoma with Brain Metastasis. Case Rep Oncol 2018;11:615-21. [Crossref] [PubMed]
- Roesel C, Kambartel K, Kopeika U, et al. Lazarus-type tumour response to therapy with nivolumab for sarcomatoid carcinomas of the lung. Curr Oncol 2019;26:e270-3. [Crossref] [PubMed]
- Qian X, Wang Y, Liu F, et al. The efficacy and safety analysis of first-line immune checkpoint inhibitors in pulmonary sarcomatoid carcinoma. Front Immunol 2022;13:956982. [Crossref] [PubMed]
- Wei JW, Hao Y, Xiang J, et al. The prognostic impact of immune checkpoint inhibitors for the treatment of pulmonary sarcomatoid carcinoma: A multicenter retrospective study. Neoplasma 2022;69:1437-44. [Crossref] [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
- Lee J, Choi Y, Jung HA, et al. Outstanding clinical efficacy of PD-1/PD-L1 inhibitors for pulmonary pleomorphic carcinoma. Eur J Cancer 2020;132:150-8. [Crossref] [PubMed]
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer 2019;7:97. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines(R) Insights: Non-Small Cell Lung Cancer, Version 2.2023. J Natl Compr Canc Netw 2023;21:340-50. [Crossref] [PubMed]


